Direct Regio- and Diastereoselective Synthesis of δ-Lactams from Acrylamides and Unactivated Alkenes Initiated by RhIII-Catalyzed C−H Activation
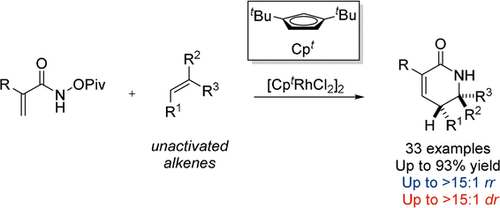
Graphical Abstract
Regio- and diastereoselective synthesis of unprotected δ-lactams has been realized starting from readily accessible acrylamides and unactivated alkenes. The reaction provides an efficient means of synthesizing a diverse set of δ-lactams in good yield and stereoselectivity, which serve as useful building blocks for substituted piperidines.
Abstract
We report a RhIII-catalyzed regio- and diastereoselective synthesis of δ-lactams from readily available acrylamide derivatives and unactivated alkenes. The reaction provides a rapid route to a diverse set of δ-lactams in good yield and stereoselectivity, which serve as useful building blocks for substituted piperidines. The regioselectivity of the reaction with unactivated terminal alkene is significantly improved by using Cpt ligand on the RhIII catalyst. The synthetic utility of the reaction is demonstrated by the preparation of a potential drug candidate containing a trisubstituted piperidine moiety. Mechanistic studies show that the reversibility of the C−H activation depends on the choice of Cp ligand on the RhIII catalyst. The irreversible C−H activation is observed and becomes turnover-limiting with [CptRhCl2]2 as catalyst.