Polyhydrazide-Based Organic Nanotubes as Efficient and Selective Artificial Iodide Channels
Dr. Arundhati Roy
NanoBio Lab, 31 Biopolis Way, The Nanos, Singapore, 138669 Singapore
These authors contributed equally to this work.
Search for more papers by this authorDr. Himanshu Joshi
Department of Physics and Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, 61801 USA
These authors contributed equally to this work.
Search for more papers by this authorRuijuan Ye
Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore, 117585 Singapore
Search for more papers by this authorDr. Jie Shen
NanoBio Lab, 31 Biopolis Way, The Nanos, Singapore, 138669 Singapore
Search for more papers by this authorDr. Feng Chen
NanoBio Lab, 31 Biopolis Way, The Nanos, Singapore, 138669 Singapore
Search for more papers by this authorCorresponding Author
Prof. Dr. Aleksei Aksimentiev
Department of Physics and Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, 61801 USA
Search for more papers by this authorCorresponding Author
Dr. Huaqiang Zeng
NanoBio Lab, 31 Biopolis Way, The Nanos, Singapore, 138669 Singapore
Search for more papers by this authorDr. Arundhati Roy
NanoBio Lab, 31 Biopolis Way, The Nanos, Singapore, 138669 Singapore
These authors contributed equally to this work.
Search for more papers by this authorDr. Himanshu Joshi
Department of Physics and Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, 61801 USA
These authors contributed equally to this work.
Search for more papers by this authorRuijuan Ye
Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore, 117585 Singapore
Search for more papers by this authorDr. Jie Shen
NanoBio Lab, 31 Biopolis Way, The Nanos, Singapore, 138669 Singapore
Search for more papers by this authorDr. Feng Chen
NanoBio Lab, 31 Biopolis Way, The Nanos, Singapore, 138669 Singapore
Search for more papers by this authorCorresponding Author
Prof. Dr. Aleksei Aksimentiev
Department of Physics and Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, 61801 USA
Search for more papers by this authorCorresponding Author
Dr. Huaqiang Zeng
NanoBio Lab, 31 Biopolis Way, The Nanos, Singapore, 138669 Singapore
Search for more papers by this authorGraphical Abstract
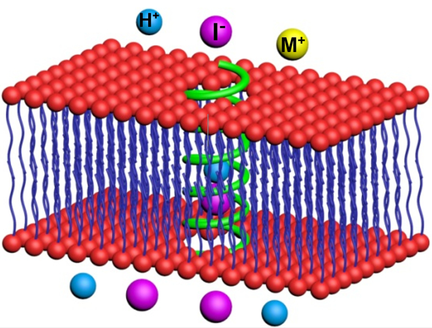
The right channels: A novel class of foldamer-based pore-forming helically folded polyhydrazides, having hydrophobic cavities of about a 6.5 Å diameter, promote transport of anions, rather than cations, across membranes, with iodide as the preferred transport species. The best channel, having a helical height of 3.6 nm, exhibits the highest recorded iodide transport activity (EC50=0.042 μm or 0.028 mol % relative to lipid) and high I−/Cl− selectivity of 11, in terms of EC50 values, or 42 based on initial rate constants.
Abstract
Reported herein is a series of pore-containing polymeric nanotubes based on a hydrogen-bonded hydrazide backbone. Nanotubes of suitable lengths, possessing a hollow cavity of about a 6.5 Å diameter, mediate highly efficient transport of diverse types of anions, rather than cations, across lipid membranes. The reported polymer channel, having an average molecular weight of 18.2 kDa and 3.6 nm in helical height, exhibits the highest anion-transport activities for iodide (EC50=0.042 μm or 0.028 mol % relative to lipid), whcih is transported 10 times more efficiently than chlorides (EC50=0.47 μm). Notably, even in cholesterol-rich environment, iodide transport activity remains high with an EC50 of 0.37 μm. Molecular dynamics simulation studies confirm that the channel is highly selective for anions and that such anion selectivity arises from a positive electrostatic potential of the central lumen rendered by the interior-pointing methyl groups.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201916287-sup-0001-misc_information.pdf3.5 MB | Supplementary |
| anie201916287-sup-0001-SM1.mp43.1 MB | Supplementary |
| anie201916287-sup-0001-SM2.mp41.7 MB | Supplementary |
| anie201916287-sup-0001-SM3.mp421.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. Duran, C. H. Thompson, Q. Xiao, H. C. Hartzell, Annu. Rev. Physiol. 2010, 72, 95–121.
- 2
- 2aF. Ahad, S. A. Ganie, Indian J. Endocrinol. Metab. 2010, 14, 13–17;
- 2bH. R. Chung, Ann. Pediatr. Endocrinol. Metab. 2014, 19, 8–12.
- 3E. Darrouzet, S. Lindenthal, D. Marcellin, J.-L. Pellequer, T. Pourcher, Biochim. Biophys. Acta Biomembr. 2014, 1838, 244–253.
- 4A. Mina, E. J. Favaloro, J. Koutts, Lab. Med. 2011, 42, 744–746.
- 5
- 5aV. Sidorov, F. W. Kotch, G. Abdrakhmanova, R. Mizani, J. C. Fettinger, J. T. Davis, J. Am. Chem. Soc. 2002, 124, 2267–2278;
- 5bP. H. Schlesinger, R. Ferdani, J. Liu, J. Pajewska, R. Pajewski, M. Saito, H. Shabany, G. W. Gokel, J. Am. Chem. Soc. 2002, 124, 1848–1849;
- 5cV. Gorteau, G. Bollot, J. Mareda, A. Perez-Velasco, S. Matile, J. Am. Chem. Soc. 2006, 128, 14788–14789;
- 5dV. Gorteau, G. Bollot, J. Mareda, S. Matile, Org. Biomol. Chem. 2007, 5, 3000–3012;
- 5eX. Li, B. Shen, X.-Q. Yao, D. Yang, J. Am. Chem. Soc. 2009, 131, 13676–13680;
- 5fC. R. Yamnitz, S. Negin, I. A. Carasel, R. K. Winter, G. W. Gokel, Chem. Commun. 2010, 46, 2838–2840;
- 5gA. Vargas Jentzsch, S. Matile, J. Am. Chem. Soc. 2013, 135, 5302–5303;
- 5hT. Saha, S. Dasari, D. Tewari, A. Prathap, K. M. Sureshan, A. K. Bera, A. Mukherjee, P. Talukdar, J. Am. Chem. Soc. 2014, 136, 14128–14135;
- 5iT. Saha, A. Gautam, A. Mukherjee, M. Lahiri, P. Talukdar, J. Am. Chem. Soc. 2016, 138, 16443–16451;
- 5jX. Wei, G. Zhang, Y. Shen, Y. Zhong, R. Liu, N. Yang, F. Y. Al-mkhaizim, M. A. Kline, L. He, M. Li, Z.-L. Lu, Z. Shao, B. Gong, J. Am. Chem. Soc. 2016, 138, 2749–2754;
- 5kS. V. Shinde, P. Talukdar, Angew. Chem. Int. Ed. 2017, 56, 4238–4242; Angew. Chem. 2017, 129, 4302–4306;
- 5lH. Behera, N. Madhavan, J. Am. Chem. Soc. 2017, 139, 12919–12922;
- 5mC. Ren, X. Ding, A. Roy, J. Shen, S. Zhou, F. Chen, S. F. Yau Li, H. Ren, Y. Y. Yang, H. Q. Zeng, Chem. Sci. 2018, 9, 4044–4051.
- 6
- 6aJ. T. Davis, O. Okunola, R. Quesada, Chem. Soc. Rev. 2010, 39, 3843–3862;
- 6bP. R. Brotherhood, A. P. Davis, Chem. Soc. Rev. 2010, 39, 3633–3647;
- 6cS. Matile, A. Vargas Jentzsch, J. Montenegro, A. Fin, Chem. Soc. Rev. 2011, 40, 2453–2474;
- 6dD. S. Kim, J. L. Sessler, Chem. Soc. Rev. 2015, 44, 532–546.
- 7
- 7aX. Wu, L. W. Judd, E. N. W. Howe, A. M. Withecombe, V. Soto-Cerrato, H. Li, N. Busschaert, H. Valkenier, R. Pérez-Tomás, D. N. Sheppard, Y.-B. Jiang, A. P. Davis, P. A. Gale, Chem 2016, 1, 127–146;
- 7bS.-K. Ko, S. K. Kim, A. Share, V. M. Lynch, J. Park, W. Namkung, W. Van Rossom, N. Busschaert, P. A. Gale, J. L. Sessler, I. Shin, Nat. Chem. 2014, 6, 885;
- 7cB. A. Smith, M. M. Daschbach, S. T. Gammon, S. Xiao, S. E. Chapman, C. Hudson, M. Suckow, D. Piwnica-Worms, G. W. Gokel, W. M. Leevy, Chem. Commun. 2011, 47, 7977–7979.
- 8
- 8aN. Madhavan, E. C. Robert, M. S. Gin, Angew. Chem. Int. Ed. 2005, 44, 7584–7587; Angew. Chem. 2005, 117, 7756–7759;
- 8bB. P. Benke, P. Aich, Y. Kim, K. L. Kim, M. R. Rohman, S. Hong, I.-C. Hwang, E. H. Lee, J. H. Roh, K. Kim, J. Am. Chem. Soc. 2017, 139, 7432–7435.
- 9J.-L. Hou, X.-B. Shao, G.-J. Chen, Y.-X. Zhou, X.-K. Jiang, Z.-T. Li, J. Am. Chem. Soc. 2004, 126, 12386–12394.
- 10P. Xin, P. Zhu, P. Su, J.-L. Hou, Z.-T. Li, J. Am. Chem. Soc. 2014, 136, 13078–13081.
- 11
- 11aD.-W. Zhang, H. Wang, Z.-T. Li, Macromol. Rapid Commun. 2017, 38, 1700179;
- 11bJ.-Y. Chen, J.-L. Hou, Org. Chem. Front. 2018, 5, 1728–1736;
- 11cF. Chen, J. Shen, N. Li, A. Roy, R. J. Ye, C. L. Ren, H. Q. Zeng, Angew. Chem. Int. Ed. 2020, 59, 1440–1444; Angew. Chem. 2020, 132, 1456–1460.
- 12D.-W. Zhang, X. Zhao, J.-L. Hou, Z.-T. Li, Chem. Rev. 2012, 112, 5271–5316.
- 13
- 13aY. Hamuro, S. J. Geib, A. D. Hamilton, Angew. Chem. Int. Ed. Engl. 1994, 33, 446; Angew. Chem. 1994, 106, 465;
- 13bJ. Zhu, R. D. Parra, H. Q. Zeng, E. Skrzypczak-Jankun, X. C. Zeng, B. Gong, J. Am. Chem. Soc. 2000, 122, 4219–4220;
- 13cV. Berl, I. Huc, R. G. Khoury, M. J. Krische, J. M. Lehn, Nature 2000, 407, 720–723.
- 14J. J. van Gorp, J. A. J. M. Vekemans, E. W. Meijer, Chem. Commun. 2004, 60–61.
- 15
- 15aBOP=((Benzotriazol-1-yloxy)tris(dimethylamino)phosphoniumhexa fluorophosphate);
- 15bZ. Y. Du, C. L. Ren, R. J. Ye, J. Shen, Y. J. Lu, J. Wang, H. Q. Zeng, Chem. Commun. 2011, 47, 12488–12490.
- 16H. Q. Zeng, R. S. Miller, R. A. Flowers, B. Gong, J. Am. Chem. Soc. 2000, 122, 2635–2644.
- 17C. Ren, J. Shen, H. Q. Zeng, J. Am. Chem. Soc. 2017, 139, 12338–12341.
- 18C. L. Ren, F. Zeng, J. Shen, F. Chen, A. Roy, S. Zhou, H. Ren, H. Q. Zeng, J. Am. Chem. Soc. 2018, 140, 8817–8826.
- 19W. Humphrey, A. Dalke, K. Schulten, J. Mol. Graph. 1996, 14, 33–38.
- 20K. Göpfrich, C.-Y. Li, I. Mames, S. P. Bhamidimarri, M. Ricci, J. Yoo, A. Mames, A. Ohmann, M. Winterhalter, E. Stulz, A. Aksimentiev, U. F. Keyser, Nano Lett. 2016, 16, 4665–4669.
- 21K. Decker, M. Page, A. Boyd, I. MacAllister, M. Ginsberg, A. Aksimentiev, ACS Biomater. Sci. Eng. 2017, 3, 342–348.
- 22There are several reasons that could explain the difference between simulated and measured currents. 1) The applied potential is typically concentrated to the transmembrane region, with limited effect on ion entrance. That is, the high bias might wash out some barriers in the middle of the channel, without affecting the barriers at the channel entrance. This scenario may explain why rectification is more pronounced at 1 V. 2) With regard to rectification, it is also possible that the terminal groups in the experiment do not maintain the same conformation as in simulations. Such partial unwinding is more likely on the experimental time scale. Without the charged group present right at the entrance of the channel, the rectification phenomenon should not happen. 3) There are potential inaccuracies regarding the channel structure and parameterization.
- 23Y. Sugita, A. Kitao, Y. Okamoto, J. Chem. Phys. 2000, 113, 6042–6051.
- 24B. Roux, Comput. Phys. Commun. 1995, 91, 275–282.
- 25C. N. Rowley, B. t. Roux, J. Chem. Theory Comput. 2012, 8, 3526–3535.
- 26A. Aksimentiev, K. Schulten, Biophys. J. 2005, 88, 3745–3761.
- 27A. Horner, F. Zocher, J. Preiner, N. Ollinger, C. Siligan, S. A. Akimov, P. Pohl, Sci. Adv. 2015, 1, e1400083.