Supramolecular Tuning Enables Selective Oxygen Reduction Catalyzed by Cobalt Porphyrins for Direct Electrosynthesis of Hydrogen Peroxide†
Peter T. Smith
Department of Chemistry, University of California, Berkeley, Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorYounghoon Kim
Department of Chemistry, Pohang University of Science and Technology, Pohang, 37673 Republic of Korea
Center for Self-assembly and Complexity (CSC), Institute for Basic Science (IBS), Pohang, 37673 Republic of Korea
Search for more papers by this authorDr. Bahiru Punja Benke
Center for Self-assembly and Complexity (CSC), Institute for Basic Science (IBS), Pohang, 37673 Republic of Korea
Search for more papers by this authorCorresponding Author
Prof. Kimoon Kim
Department of Chemistry, Pohang University of Science and Technology, Pohang, 37673 Republic of Korea
Center for Self-assembly and Complexity (CSC), Institute for Basic Science (IBS), Pohang, 37673 Republic of Korea
Search for more papers by this authorCorresponding Author
Prof. Christopher J. Chang
Department of Chemistry, University of California, Berkeley, Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorPeter T. Smith
Department of Chemistry, University of California, Berkeley, Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorYounghoon Kim
Department of Chemistry, Pohang University of Science and Technology, Pohang, 37673 Republic of Korea
Center for Self-assembly and Complexity (CSC), Institute for Basic Science (IBS), Pohang, 37673 Republic of Korea
Search for more papers by this authorDr. Bahiru Punja Benke
Center for Self-assembly and Complexity (CSC), Institute for Basic Science (IBS), Pohang, 37673 Republic of Korea
Search for more papers by this authorCorresponding Author
Prof. Kimoon Kim
Department of Chemistry, Pohang University of Science and Technology, Pohang, 37673 Republic of Korea
Center for Self-assembly and Complexity (CSC), Institute for Basic Science (IBS), Pohang, 37673 Republic of Korea
Search for more papers by this authorCorresponding Author
Prof. Christopher J. Chang
Department of Chemistry, University of California, Berkeley, Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA, 94720-1460 USA
Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA, 94720-1460 USA
Search for more papers by this authorA previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv.11401359.v1).
Graphical Abstract
Abstract
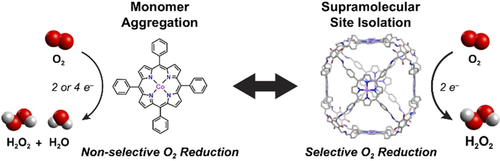
We report a supramolecular strategy for promoting the selective reduction of O2 for direct electrosynthesis of H2O2. We utilized cobalt tetraphenylporphyrin (Co-TPP), an oxygen reduction reaction (ORR) catalyst with highly variable product selectivity, as a building block to assemble the permanently porous supramolecular cage Co-PB-1(6) bearing six Co-TPP subunits connected through twenty-four imine bonds. Reduction of these imine linkers to amines yields the more flexible cage Co-rPB-1(6). Both Co-PB-1(6) and Co-rPB-1(6) cages produce 90–100 % H2O2 from electrochemical ORR catalysis in neutral pH water, whereas the Co-TPP monomer gives a 50 % mixture of H2O2 and H2O. Bimolecular pathways have been implicated in facilitating H2O formation, therefore, we attribute this high H2O2 selectivity to site isolation of the discrete molecular units in each supramolecule. The ability to control reaction selectivity in supramolecular structures beyond traditional host–guest interactions offers new opportunities for designing such architectures for a broader range of catalytic applications.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201916131-sup-0001-misc_information.pdf5.1 MB | Supplementary |
| anie201916131-sup-0001-Movie_1_PB6.mov27.2 MB | Supplementary |
| anie201916131-sup-0001-Movie_2_rPB6.mov27.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Ferguson-Miller, G. T. Babcock, Chem. Rev. 1996, 96, 2889–2908;
- 1bP. Brzezinski, R. B. Gennis, J. Bioenerg. Biomembr. 2008, 40, 521–531;
- 1cL. Dai, Y. Xue, L. Qu, H. J. Choi, J. B. Baek, Chem. Rev. 2015, 115, 4823–4892;
- 1dM. L. Pegis, C. F. Wise, D. J. Martin, J. M. Mayer, Chem. Rev. 2018, 118, 2340–2391.
- 2
- 2aJ. P. Collman, N. K. Devaraj, R. A. Decreau, Y. Yang, Y. L. Yan, W. Ebina, T. A. Eberspacher, C. E. Chidsey, Science 2007, 315, 1565–1568;
- 2bJ. Rosenthal, D. G. Nocera, Acc. Chem. Res. 2007, 40, 543–553;
- 2cC. Costentin, H. Dridi, J. M. Saveant, J. Am. Chem. Soc. 2015, 137, 13535–13544;
- 2dS. Chatterjee, K. Sengupta, B. Mondal, S. Dey, A. Dey, Acc. Chem. Res. 2017, 50, 1744–1753;
- 2eY. H. Wang, P. E. Schneider, Z. K. Goldsmith, B. Mondal, S. Hammes-Schiffer, S. S. Stahl, ACS Cent. Sci. 2019, 5, 1024–1034.
- 3
- 3aJ. M. Campos-Martin, G. Blanco-Brieva, J. L. Fierro, Angew. Chem. Int. Ed. 2006, 45, 6962–6984; Angew. Chem. 2006, 118, 7116–7139;
- 3bI. Yamanaka, T. Murayama, Angew. Chem. Int. Ed. 2008, 47, 1900–1902; Angew. Chem. 2008, 120, 1926–1928;
- 3cS. J. Freakley, Q. He, J. H. Harrhy, L. Lu, D. A. Crole, D. J. Morgan, E. N. Ntainjua, J. K. Edwards, A. F. Carley, A. Y. Borisevich, C. J. Kiely, G. J. Hutchings, Science 2016, 351, 965–968;
- 3dH. W. Kim, M. B. Ross, N. Kornienko, L. Zhang, J. H. Guo, P. D. Yang, B. D. McCloskey, Nat. Catal. 2018, 1, 282–290;
- 3eS. C. Perry, D. Pangotra, L. Vieira, L. I. Csepei, V. Sieber, L. Wang, C. P. de Leon, F. C. Walsh, Nat. Rev. Chem. 2019, 3, 442–458;
- 3fC. Xia, Y. Xia, P. Zhu, L. Fan, H. Wang, Science 2019, 366, 226–231.
- 4
- 4aJ. P. Collman, P. Denisevich, Y. Konai, M. Marrocco, C. Koval, F. C. Anson, J. Am. Chem. Soc. 1980, 102, 6027–6036;
- 4bC. J. Chang, Y. Q. Deng, C. N. Shi, C. K. Chang, F. C. Anson, D. G. Nocera, Chem. Commun. 2000, 1355–1356;
- 4cC. J. Chang, Z. H. Loh, C. Shi, F. C. Anson, D. G. Nocera, J. Am. Chem. Soc. 2004, 126, 10013–10020;
- 4dK. M. Kadish, L. Fremond, Z. Ou, J. Shao, C. Shi, F. C. Anson, F. Burdet, C. P. Gros, J. M. Barbe, R. Guilard, J. Am. Chem. Soc. 2005, 127, 5625–5631;
- 4eG. Passard, A. M. Ullman, C. N. Brodsky, D. G. Nocera, J. Am. Chem. Soc. 2016, 138, 2925–2928;
- 4fN. Mihara, Y. Yamada, H. Takaya, Y. Kitagawa, S. Aoyama, K. Igawa, K. Tomooka, K. Tanaka, Chem. Eur. J. 2017, 23, 7508–7514;
- 4gA. N. Oldacre, M. R. Crawley, A. E. Friedman, T. R. Cook, Chem. Eur. J. 2018, 24, 10984–10987.
- 5
- 5aR. McGuire, Jr., D. K. Dogutan, T. S. Teets, J. Suntivich, Y. Shao-Horn, D. G. Nocera, Chem. Sci. 2010, 1, 411–414;
- 5bR. L. Shook, S. M. Peterson, J. Greaves, C. Moore, A. L. Rheingold, A. S. Borovik, J. Am. Chem. Soc. 2011, 133, 5810–5817;
- 5cC. T. Carver, B. D. Matson, J. M. Mayer, J. Am. Chem. Soc. 2012, 134, 5444–5447;
- 5dS. Bhunia, A. Rana, P. Roy, D. J. Martin, M. L. Pegis, B. Roy, A. Dey, J. Am. Chem. Soc. 2018, 140, 9444–9457;
- 5eS. Sinha, M. Ghosh, J. J. Warren, ACS Catal. 2019, 9, 2685–2691.
- 6
- 6aC. Shi, F. C. Anson, Inorg. Chem. 1992, 31, 5078–5083;
- 6bK. Mittra, S. Chatterjee, S. Samanta, A. Dey, Inorg. Chem. 2013, 52, 14317–14325.
- 7
- 7aT. Geiger, F. C. Anson, J. Am. Chem. Soc. 1981, 103, 7489–7496;
- 7bR. J. H. Chan, Y. O. Su, T. Kuwana, Inorg. Chem. 1985, 24, 3777–3784;
- 7cB. Sun, Z. Ou, D. Meng, Y. Fang, Y. Song, W. Zhu, P. V. Solntsev, V. N. Nemykin, K. M. Kadish, Inorg. Chem. 2014, 53, 8600–8609;
- 7dW. Schöfberger, F. Faschinger, S. Chattopadhyay, S. Bhakta, B. Mondal, J. A. Elemans, S. Mullegger, S. Tebi, R. Koch, F. Klappenberger, M. Paszkiewicz, J. V. Barth, E. Rauls, H. Aldahhak, W. G. Schmidt, A. Dey, Angew. Chem. Int. Ed. 2016, 55, 2350–2355; Angew. Chem. 2016, 128, 2396–2401;
- 7eY. H. Wang, M. L. Pegis, J. M. Mayer, S. S. Stahl, J. Am. Chem. Soc. 2017, 139, 16458–16461;
- 7fS. L. Hooe, A. L. Rheingold, C. W. Machan, J. Am. Chem. Soc. 2018, 140, 3232–3241.
- 8
- 8aJ. P. Collman, M. Rapta, M. Bröring, L. Raptova, R. Schwenninger, B. Boitrel, L. Fu, M. L′Her, J. Am. Chem. Soc. 1999, 121, 1387–1388;
- 8bS. Mukherjee, A. Mukherjee, A. Bhagi-Damodaran, M. Mukherjee, Y. Lu, A. Dey, Nat. Commun. 2015, 6, 8467;
- 8cC. Liu, H. Lei, Z. Zhang, F. Chen, R. Cao, Chem. Commun. 2017, 53, 3189–3192;
- 8dL. E. Lieske, S. L. Hooe, A. W. Nichols, C. W. Machan, Dalton Trans. 2019, 48, 8633–8641.
- 9
- 9aP. Ballester, M. Fujita, J. Rebek, Jr., Chem. Soc. Rev. 2015, 44, 392–393;
- 9bC. J. Brown, F. D. Toste, R. G. Bergman, K. N. Raymond, Chem. Rev. 2015, 115, 3012–3035.
- 10
- 10aC. Shi, F. C. Anson, Inorg. Chem. 1998, 37, 1037–1043;
- 10bJ. Y. Qu, S. Yan, X. H. Qu, S. J. Dong, Electroanalysis 2004, 16, 1444–1450;
- 10cI. Hatay, B. Su, F. Li, M. A. Mendez, T. Khoury, C. P. Gros, J. M. Barbe, M. Ersoz, Z. Samec, H. H. Girault, J. Am. Chem. Soc. 2009, 131, 13453–13459;
- 10dC. C. McCrory, A. Devadoss, X. Ottenwaelder, R. D. Lowe, T. D. Stack, C. E. Chidsey, J. Am. Chem. Soc. 2011, 133, 3696–3699.
- 11
- 11aS. Siahrostami, A. Verdaguer-Casadevall, M. Karamad, D. Deiana, P. Malacrida, B. Wickman, M. Escudero-Escribano, E. A. Paoli, R. Frydendal, T. W. Hansen, I. Chorkendorff, I. E. Stephens, J. Rossmeisl, Nat. Mater. 2013, 12, 1137–1143;
- 11bK. Jiang, S. Back, A. J. Akey, C. Xia, Y. Hu, W. Liang, D. Schaak, E. Stavitski, J. K. Norskov, S. Siahrostami, H. Wang, Nat. Commun. 2019, 10, 3997.
- 12
- 12aE. M. Miner, S. Gul, N. D. Ricke, E. Pastor, J. Yano, V. K. Yachandra, T. Van Voorhis, M. Dincă, ACS Catal. 2017, 7, 7726–7731;
- 12bM. Lions, J. B. Tommasino, R. Chattot, B. Abeykoon, N. Guillou, T. Devic, A. Demessence, L. Cardenas, F. Maillard, A. Fateeva, Chem. Commun. 2017, 53, 6496–6499;
- 12cL. Z. Peng, P. Liu, Q. Q. Cheng, W. J. Hu, Y. A. Liu, J. S. Li, B. Jiang, X. S. Jia, H. Yang, K. Wen, Chem. Commun. 2018, 54, 4433–4436.
- 13C. J. Kaminsky, J. Wright, Y. Surendranath, ACS Catal. 2019, 9, 3667–3671.
- 14A. T. Murray, S. Voskian, M. Schreier, T. A. Hatton, Y. Surendranath, Joule 2019, 3, 2942–2954.
- 15
- 15aG. Zhang, M. Mastalerz, Chem. Soc. Rev. 2014, 43, 1934–1947;
- 15bA. I. Cooper, ACS Cent. Sci. 2017, 3, 544–553.
- 16A. Kewley, A. Stephenson, L. J. Chen, M. E. Briggs, T. Hasell, A. I. Cooper, Chem. Mater. 2015, 27, 3207–3210.
- 17B. P. Benke, P. Aich, Y. Kim, K. L. Kim, M. R. Rohman, S. Hong, I. C. Hwang, E. H. Lee, J. H. Roh, K. Kim, J. Am. Chem. Soc. 2017, 139, 7432–7435.
- 18N. Sun, C. Wang, H. Wang, L. Yang, P. Jin, W. Zhang, J. Jiang, Angew. Chem. Int. Ed. 2019, 58, 18011–18016; Angew. Chem. 2019, 131, 18179–18184.
- 19A. Petronico, T. P. Moneypenny II, B. G. Nicolau, J. S. Moore, R. G. Nuzzo, A. A. Gewirth, J. Am. Chem. Soc. 2018, 140, 7504–7509.
- 20R. Djemili, L. Kocher, S. Durot, A. Peuronen, K. Rissanen, V. Heitz, Chem. Eur. J. 2019, 25, 1481–1487.
- 21
- 21aM. W. Schneider, I. M. Oppel, A. Griffin, M. Mastalerz, Angew. Chem. Int. Ed. 2013, 52, 3611–3615; Angew. Chem. 2013, 125, 3699–3703;
- 21bJ. L. Culshaw, G. Cheng, M. Schmidtmann, T. Hasell, M. Liu, D. J. Adams, A. I. Cooper, J. Am. Chem. Soc. 2013, 135, 10007–10010;
- 21cT. H. G. Schick, J. C. Lauer, F. Rominger, M. Mastalerz, Angew. Chem. Int. Ed. 2019, 58, 1768–1773; Angew. Chem. 2019, 131, 1782–1787.
- 22M. Brutschy, M. W. Schneider, M. Mastalerz, S. R. Waldvogel, Chem. Commun. 2013, 49, 8398–8400.
- 23M. Liu, L. Zhang, M. A. Little, V. Kapil, M. Ceriotti, S. Yang, L. Ding, D. L. Holden, R. Balderas-Xicohtencatl, D. He, R. Clowes, S. Y. Chong, G. Schutz, L. Chen, M. Hirscher, A. I. Cooper, Science 2019, 366, 613–620.
- 24
- 24aV. S. Thoi, Y. Sun, J. R. Long, C. J. Chang, Chem. Soc. Rev. 2013, 42, 2388–2400;
- 24bD. Z. Zee, T. Chantarojsiri, J. R. Long, C. J. Chang, Acc. Chem. Res. 2015, 48, 2027–2036;
- 24cP. T. Smith, E. M. Nichols, Z. Cao, C. J. Chang, submitted 2019.
- 25
- 25aS. Hong, M. R. Rohman, J. Jia, Y. Kim, D. Moon, Y. Kim, Y. H. Ko, E. Lee, K. Kim, Angew. Chem. Int. Ed. 2015, 54, 13241–13244; Angew. Chem. 2015, 127, 13439–13442;
- 25bY. Kim, J. Koo, I. C. Hwang, R. D. Mukhopadhyay, S. Hong, J. Yoo, A. A. Dar, I. Kim, D. Moon, T. J. Shin, Y. H. Ko, K. Kim, J. Am. Chem. Soc. 2018, 140, 14547–14551;
- 25cR. D. Mukhopadhyay, Y. Kim, J. Koo, K. Kim, Acc. Chem. Res. 2018, 51, 2730–2738.
- 26P. T. Smith, B. P. Benke, Z. Cao, Y. Kim, E. M. Nichols, K. Kim, C. J. Chang, Angew. Chem. Int. Ed. 2018, 57, 9684–9688; Angew. Chem. 2018, 130, 9832–9836.
- 27
- 27aY. Jin, B. A. Voss, R. D. Noble, W. Zhang, Angew. Chem. Int. Ed. 2010, 49, 6348–6351; Angew. Chem. 2010, 122, 6492–6495;
- 27bM. Mastalerz, M. W. Schneider, I. M. Oppel, O. Presly, Angew. Chem. Int. Ed. 2011, 50, 1046–1051; Angew. Chem. 2011, 123, 1078–1083;
- 27cM. Liu, M. A. Little, K. E. Jelfs, J. T. Jones, M. Schmidtmann, S. Y. Chong, T. Hasell, A. I. Cooper, J. Am. Chem. Soc. 2014, 136, 7583–7586.
- 28A. Maurin, M. Robert, J. Am. Chem. Soc. 2016, 138, 2492–2495.
- 29
- 29aS. Lin, C. S. Diercks, Y. B. Zhang, N. Kornienko, E. M. Nichols, Y. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi, C. J. Chang, Science 2015, 349, 1208–1213;
- 29bA. Zhanaidarova, S. C. Jones, E. Despagnet-Ayoub, B. R. Pimentel, C. P. Kubiak, J. Am. Chem. Soc. 2019, 141, 17270–17277.
- 30S. G. Bratsch, J. Phys. Chem. Ref. Data 1989, 18, 1–21.
- 31A. N. Oldacre, A. E. Friedman, T. R. Cook, J. Am. Chem. Soc. 2017, 139, 1424–1427.
- 32M. L. Rigsby, D. J. Wasylenko, M. L. Pegis, J. M. Mayer, J. Am. Chem. Soc. 2015, 137, 4296–4299.
- 33R. F. Zhou, Y. Zheng, M. Jaroniec, S. Z. Qiao, ACS Catal. 2016, 6, 4720–4728.