Homogeneous, Low-volume, Efficient, and Sensitive Quantitation of Circulating Exosomal PD-L1 for Cancer Diagnosis and Immunotherapy Response Prediction
Mengjiao Huang
The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, the Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorDr. Juanjuan Yang
College of Biological Science and Engineering, Fuzhou University, Fuzhou, 350002 China
Search for more papers by this authorTeng Wang
College of Biological Science and Engineering, Fuzhou University, Fuzhou, 350002 China
Search for more papers by this authorDr. Jia Song
Institute of Molecular Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
Search for more papers by this authorJinglu Xia
College of Biological Science and Engineering, Fuzhou University, Fuzhou, 350002 China
Search for more papers by this authorDr. Lingling Wu
Institute of Molecular Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
Search for more papers by this authorDr. Wei Wang
Institute of Molecular Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
Search for more papers by this authorDr. Qiaoyi Wu
The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, the Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorProf. Zhi Zhu
The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, the Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorCorresponding Author
Dr. Yanling Song
The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, the Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Institute of Molecular Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
Search for more papers by this authorCorresponding Author
Prof. Chaoyong Yang
The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, the Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Institute of Molecular Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
Search for more papers by this authorMengjiao Huang
The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, the Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorDr. Juanjuan Yang
College of Biological Science and Engineering, Fuzhou University, Fuzhou, 350002 China
Search for more papers by this authorTeng Wang
College of Biological Science and Engineering, Fuzhou University, Fuzhou, 350002 China
Search for more papers by this authorDr. Jia Song
Institute of Molecular Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
Search for more papers by this authorJinglu Xia
College of Biological Science and Engineering, Fuzhou University, Fuzhou, 350002 China
Search for more papers by this authorDr. Lingling Wu
Institute of Molecular Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
Search for more papers by this authorDr. Wei Wang
Institute of Molecular Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
Search for more papers by this authorDr. Qiaoyi Wu
The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, the Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorProf. Zhi Zhu
The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, the Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Search for more papers by this authorCorresponding Author
Dr. Yanling Song
The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, the Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Institute of Molecular Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
Search for more papers by this authorCorresponding Author
Prof. Chaoyong Yang
The MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, the Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, Department of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 China
Institute of Molecular Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200127 China
Search for more papers by this authorGraphical Abstract
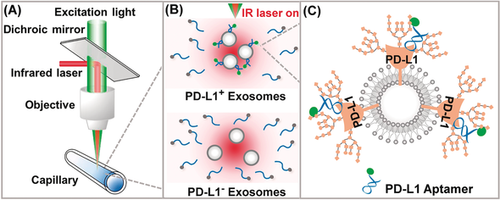
An aptamer-induced thermophoresis quantitation of exosomal programmed death-ligand 1 (PD-L1, a transmembrane protein) was developed, which integrates effective recognition of aptamer and homogeneous thermophoresis. The facile technique is more sensitive and efficient than the current enzyme-linked immunosorbent assay (ELISA)-based methods. Translation of the method into standard clinical practice for immunotherapy prediction and monitoring is anticipated.
Abstract
Immunotherapy has revolutionized cancer treatment, but its efficacy is severely hindered by the lack of effective predictors. Herein, we developed a homogeneous, low-volume, efficient, and sensitive exosomal programmed death-ligand 1 (PD-L1, a type of transmembrane protein) quantitation method for cancer diagnosis and immunotherapy response prediction (HOLMES-ExoPD-L1). The method combines a newly evolved aptamer that efficiently binds to PD-L1 with less hindrance by antigen glycosylation than antibody, and homogeneous thermophoresis with a rapid binding kinetic. As a result, HOLMES-ExoPD-L1 is higher in sensitivity, more rapid in reaction time, and easier to operate than existing enzyme-linked immunosorbent assay (ELISA)-based methods. As a consequence of an outstanding improvement of sensitivity, the level of circulating exosomal PD-L1 detected by HOLMES-ExoPD-L1 can effectively distinguish cancer patients from healthy volunteers, and for the first time was found to correlate positively with the metastasis of adenocarcinoma. Overall, HOLMES-ExoPD-L1 brings a fresh approach to exosomal PD-L1 quantitation, offering unprecedented potential for early cancer diagnosis and immunotherapy response prediction.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201916039-sup-0001-misc_information.pdf3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. Chen, X. Han, J. Clin. Invest. 2015, 125, 3384–3391;
- 1bP. Sharma, J. P. Allison, Science 2015, 348, 56–61.
- 2
- 2aA. M. M. Eggermont, C. U. Blank, M. Mandala, G. V. Long, V. Atkinson, S. Dalle, A. Haydon, M. Lichinitser, A. Khattak, M. S. Carlino, S. Sandhu, J. Larkin, S. Puig, P. A. Ascierto, P. Rutkowski, D. Schadendorf, R. Koornstra, L. Hernandez-Aya, M. Maio, A. J. M. van den Eertwegh, J.-J. Grob, R. Gutzmer, R. Jamal, P. Lorigan, N. Ibrahim, S. Marreaud, A. C. J. van Akkooi, S. Suciu, C. Robert, N. Engl. J. Med. 2018, 378, 1789–1801;
- 2bL. Fehrenbacher, A. Spira, M. Ballinger, M. Kowanetz, J. Vansteenkiste, J. Mazieres, K. Park, D. Smith, A. Artal-Cortes, C. Lewanski, F. Braiteh, D. Waterkamp, P. He, W. Zou, D. S. Chen, J. Yi, A. Sandler, A. Rittmeyer, Lancet 2016, 387, 1837–1846.
- 3A. M. Goodman, S. Kato, L. Bazhenova, S. P. Patel, G. M. Frampton, V. Miller, P. J. Stephens, G. A. Daniels, R. Kurzrock, Mol. Cancer Ther. 2017, 16, 2598–2608.
- 4P. Jiang, S. Gu, D. Pan, J. Fu, A. Sahu, X. Hu, Z. Li, N. Traugh, X. Bu, B. Li, J. Liu, G. J. Freeman, M. A. Brown, K. W. Wucherpfennig, X. S. Liu, Nat. Med. 2018, 24, 1550–1558.
- 5C. Krieg, M. Nowicka, S. Guglietta, S. Schindler, F. J. Hartmann, L. M. Weber, R. Dummer, M. D. Robinson, M. P. Levesque, B. Becher, Nat. Med. 2018, 24, 144–153.
- 6
- 6aG. Chen, A. C. Huang, W. Zhang, G. Zhang, M. Wu, W. Xu, Z. Yu, J. Yang, B. Wang, H. Sun, H. Xia, Q. Man, W. Zhong, L. F. Antelo, B. Wu, X. Xiong, X. Liu, L. Guan, T. Li, S. Liu, R. Yang, Y. Lu, L. Dong, S. McGettigan, R. Somasundaram, R. Radhakrishnan, G. Mills, Y. Lu, J. Kim, Y. H. Chen, H. Dong, Y. Zhao, G. C. Karakousis, T. C. Mitchell, L. M. Schuchter, M. Herlyn, E. J. Wherry, X. Xu, W. Guo, Nature 2018, 560, 382–386;
- 6bM. Poggio, T. Hu, C.-C. Pai, B. Chu, C. D. Belair, A. Chang, E. Montabana, U. E. Lang, Q. Fu, L. Fong, R. Blelloch, Cell 2019, 177, 414–427.
- 7Y. Song, L. Wu, C. Yang, Natl. Sci. Rev. 2018, https://doi.org/10.1093/nsr/nwy154.
- 8
- 8aX. Gao, S. Li, F. Ding, H. Fan, L. Shi, L. Zhu, J. Li, J. Feng, X. Zhu, C. Zhang, Angew. Chem. Int. Ed. 2019, 58, 8719–8723; Angew. Chem. 2019, 131, 8811–8815;
- 8bK. Boriachek, M. K. Masud, C. Palma, H.-P. Phan, Y. Yamauchi, M. S. A. Hossain, N.-T. Nguyen, C. Salomon, M. J. A. Shiddiky, Anal. Chem. 2019, 91, 3827–3834;
- 8cA. A. I. Sina, R. Vaidyanathan, A. Wuethrich, L. G. Carrascosa, M. Trau, Anal. Bioanal. Chem. 2019, 411, 1311–1318;
- 8dR. Jauregui, S. Srinivasan, L. N. Vojtech, H. S. Gammill, D. T. Chiu, F. Hladik, P. S. Stayton, J. J. Lai, ACS Appl. Mater. Interfaces 2018, 10, 33847–33856;
- 8eY. Jiang, M. Shi, Y. Liu, S. Wan, C. Cui, L. Zhang, W. Tan, Angew. Chem. Int. Ed. 2017, 56, 11916–11920; Angew. Chem. 2017, 129, 12078–12082.
- 9
- 9aR. Vaidyanathan, M. Naghibosadat, S. Rauf, D. Korbie, L. G. Carrascosa, M. J. A. Shiddiky, M. Trau, Anal. Chem. 2014, 86, 11125–11132;
- 9bC. Liu, J. Zhao, F. Tian, J. Chang, W. Zhang, J. Sun, J. Am. Chem. Soc. 2019, 141, 3817–3821;
- 9cP. Zhang, X. Zhou, M. He, Y. Shang, A. L. Tetlow, A. K. Godwin, Y. Zeng, Nat. Biomed. Eng. 2019, 3, 438–451.
- 10C.-W. Li, S.-O. Lim, E. M. Chung, Y.-S. Kim, A. H. Park, J. Yao, J.-H. Cha, W. Xia, L.-C. Chan, T. Kim, S.-S. Chang, H.-H. Lee, C.-K. Chou, Y.-L. Liu, H.-C. Yeh, E. P. Perillo, A. K. Dunn, C.-W. Kuo, K.-H. Khoo, J. L. Hsu, Y. Wu, J.-M. Hsu, H. Yamaguchi, T.-H. Huang, A. A. Sahin, G. N. Hortobagyi, S. S. Yoo, M.-C. Hung, Cancer Cell 2018, 33, 187–201.
- 11H.-H. Lee, Y.-N. Wang, W. Xia, C.-H. Chen, K.-M. Rau, L. Ye, Y. Wei, C.-K. Chou, S.-C. Wang, M. Yan, C.-Y. Tu, T.-C. Hsia, S.-F. Chiang, K. S. C. Chao, I. I. Wistuba, J. L. Hsu, G. N. Hortobagyi, M.-C. Hung, Cancer Cell 2019, 36, 168–178.
- 12
- 12aX. H. Fang, W. H. Tan, Acc. Chem. Res. 2010, 43, 48–57;
- 12bN. Zhang, T. Bing, L. Y. Shen, R. S. Song, L. L. Wang, X. J. Liu, M. R. Liu, J. Li, W. H. Tan, D. H. Shangguan, Angew. Chem. Int. Ed. 2016, 55, 3914–3918; Angew. Chem. 2016, 128, 3982–3986;
- 12cF. Zhou, P. Wang, Y. B. Peng, P. G. Zhang, Q. Huang, W. D. Sun, N. Y. He, T. Fu, Z. L. Zhao, X. H. Fang, W. H. Tan, Angew. Chem. Int. Ed. 2019, 58, 11661–11665; Angew. Chem. 2019, 131, 11787–11791.
- 13
- 13aP. Baaske, C. J. Wienken, P. Reineck, S. Duhr, D. Braun, Angew. Chem. Int. Ed. 2010, 49, 2238–2241; Angew. Chem. 2010, 122, 2286–2290;
- 13bS. A. I. Seidel, C. J. Wienken, S. Geissler, M. Jerabek-Willemsen, S. Duhr, A. Reiter, D. Trauner, D. Braun, P. Baaske, Angew. Chem. Int. Ed. 2012, 51, 10656–10659; Angew. Chem. 2012, 124, 10810–10814;
- 13cC. Liu, J. Zhao, F. Tian, L. Cai, W. Zhang, Q. Feng, J. Chang, F. Wan, Y. Yang, B. Dai, Y. Cong, B. Ding, J. Sun, W. Tan, Nat. Biomed. Eng. 2019, 3, 183–193.
- 14C. J. Wienken, P. Baaske, U. Rothbauer, D. Braun, S. Duhr, Nat. Commun. 2010, 1, 100.
- 15S. A. I. Seidel, N. A. Markwardt, S. A. Lanzmich, D. Braun, Angew. Chem. Int. Ed. 2014, 53, 7948–7951; Angew. Chem. 2014, 126, 8082–8086.
- 16P. Reineck, C. J. Wienken, D. Braun, Electrophoresis 2010, 31, 279–286.
- 17
- 17aR. Yazdian-Robati, M. Ramezani, M. Khedri, N. Ansari, K. Abnous, S. M. Taghdisi, Microchim. Acta 2017, 184, 4029–4035;
- 17bW. Y. Lai, B. T. Huang, J. W. Wang, P. Y. Lin, P. C. Yang, Mol. Ther. Nucleic Acids 2016, 5, e397.
- 18C.-W. Li, S.-O. Lim, W. Xia, H.-H. Lee, L.-C. Chan, C.-W. Kuo, K.-H. Khoo, S.-S. Chang, J.-H. Cha, T. Kim, J. L. Hsu, Y. Wu, J.-M. Hsu, H. Yamaguchi, Q. Ding, Y. Wang, J. Yao, C.-C. Lee, H.-J. Wu, A. A. Sahin, J. P. Allison, D. Yu, G. N. Hortobagyi, M.-C. Hung, Nat. Commun. 2016, 7, 12632.
- 19S. Wan, L. Q. Zhang, S. Wang, Y. Liu, C. C. Wu, C. Cui, H. Sun, M. L. Shi, Y. Jiang, L. Li, L. P. Qiu, W. H. Tan, J. Am. Chem. Soc. 2017, 139, 5289–5292.
- 20M. N. Theodoraki, S. S. Yerneni, T. K. Hoffmann, W. E. Gooding, T. L. Whiteside, Clin. Cancer Res. 2018, 24, 896–905.