Rational Development of Remote C−H Functionalization of Biphenyl: Experimental and Computational Studies
Dr. Zhoulong Fan
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
These authors contributed equally to this work.
Search for more papers by this authorKatherine L. Bay
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA, 90095 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Xiangyang Chen
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA, 90095 USA
Search for more papers by this authorZhe Zhuang
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorHan Seul Park
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Kap-Sun Yeung
Discovery Chemistry, Bristol-Myers Squibb Research and Development, 100 Binney Street, Cambridge, MA, 02142 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. K. N. Houk
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA, 90095 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Quan Yu
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Zhoulong Fan
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
These authors contributed equally to this work.
Search for more papers by this authorKatherine L. Bay
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA, 90095 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Xiangyang Chen
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA, 90095 USA
Search for more papers by this authorZhe Zhuang
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorHan Seul Park
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Kap-Sun Yeung
Discovery Chemistry, Bristol-Myers Squibb Research and Development, 100 Binney Street, Cambridge, MA, 02142 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. K. N. Houk
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA, 90095 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Quan Yu
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorGraphical Abstract
Abstract
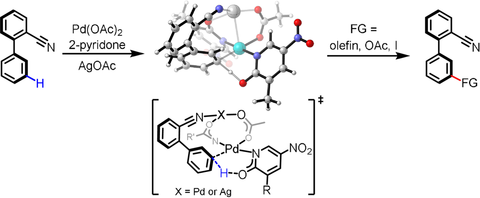
A simple and efficient nitrile-directed meta-C−H olefination, acetoxylation, and iodination of biaryl compounds is reported. Compared to the previous approach of installing a complex U-shaped template to achieve a molecular U-turn and assemble the large-sized cyclophane transition state for the remote C−H activation, a synthetically useful phenyl nitrile functional group could also direct remote meta-C−H activation. This reaction provides a useful method for the modification of biaryl compounds because the nitrile group can be readily converted to amines, acids, amides, or other heterocycles. Notably, the remote meta-selectivity of biphenylnitriles could not be expected from previous results with a macrocyclophane nitrile template. DFT computational studies show that a ligand-containing Pd–Ag heterodimeric transition state (TS) favors the desired remote meta-selectivity. Control experiments demonstrate the directing effect of the nitrile group and exclude the possibility of non-directed meta-C−H activation. Substituted 2-pyridone ligands were found to be key in assisting the cleavage of the meta-C−H bond in the concerted metalation–deprotonation (CMD) process.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201915624-sup-0001-misc_information.pdf4.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. Breslow, M. A. Winnik, J. Am. Chem. Soc. 1969, 91, 3083;
- 1bR. Breslow, R. J. Corcoran, B. B. Snider, J. Am. Chem. Soc. 1974, 96, 6791;
- 1cR. Breslow, Acc. Chem. Res. 1980, 13, 170.
- 2For other examples of site-selective C−H activation, see:
- 2aY.-H. Zhang, B.-F. Shi, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 5072;
- 2bC. Cheng, J. F. Hartwig, Science 2014, 343, 853;
- 2cR. J. Phipps, M. J. Gaunt, Science 2009, 323, 1593;
- 2dY. Yang, R. Li, Y. Zhao, D. Zhao, Z. Shi, J. Am. Chem. Soc. 2016, 138, 8734;
- 2eY. Kuninobu, H. Ida, M. Nishi, M. Kanai, Nat. Chem. 2015, 7, 712;
- 2fH. J. Davis, M. T. Mihai, R. J. Phipps, J. Am. Chem. Soc. 2016, 138, 12759;
- 2gX.-C. Wang, W. Gong, L.-Z. Fang, R.-Y. Zhu, S. Li, K. M. Engle, J.-Q. Yu, Nature 2015, 519, 334;
- 2hZ. Dong, J. Wang, G. Dong, J. Am. Chem. Soc. 2015, 137, 5887;
- 2iO. Saidi, J. Marafie, A. E. Ledger, P. M. Liu, M. F. Mahon, G. Kociok-Köhn, M. K. Whittlesey, C. G. Frost, J. Am. Chem. Soc. 2011, 133, 19298;
- 2jN. Hofmann, L. Ackermann, J. Am. Chem. Soc. 2013, 135, 5877.
- 3D. Leow, G. Li, T.-S. Mei, J.-Q. Yu, Nature 2012, 486, 518.
- 4For examples of nitrile-template-directed meta- or para- C−H activation, see:
- 4aH.-X. Dai, G. Li, X.-G. Zhang, A. F. Stepan, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 7567;
- 4bS. Lee, H. Lee, K. L. Tan, J. Am. Chem. Soc. 2013, 135, 18778;
- 4cL. Wan, N. Dastbaravardeh, G. Li, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 18056;
- 4dR.-Y. Tang, G. Li, J.-Q. Yu, Nature 2014, 507, 215;
- 4eG. Yang, P. Lindovska, D. Zhu, J. Kim, P. Wang, R.-Y. Tang, M. Movassaghi, J.-Q. Yu, J. Am. Chem. Soc. 2014, 136, 10807;
- 4fS. Bag, T. Patra, A. Modak, A. Deb, S. Maity, U. Dutta, A. Dey, R. Kancherla, A. Maji, A. Hazra, M. Bera, D. Maiti, J. Am. Chem. Soc. 2015, 137, 11888;
- 4gS. Li, L. Cai, H. Ji, L. Yang, G. Li, Nat. Commun. 2016, 7, 10443;
- 4hA. Maji, S. Guin, S. Feng, A. Dahiya, V. K. Singh, P. Liu, D. Maiti, Angew. Chem. Int. Ed. 2017, 56, 14903; Angew. Chem. 2017, 129, 15099;
- 4iA. Modak, T. Patra, R. Chowdhury, S. Raul, D. Maiti, Organometallics 2017, 36, 2418;
- 4jL. Zhang, C. Zhao, Y. Liu, J. Xu, X. Xu, Z. Jin, Angew. Chem. Int. Ed. 2017, 56, 12245; Angew. Chem. 2017, 129, 12413;
- 4kM. Li, M. Shang, H. Xu, X. Wang, H.-X. Dai, J.-Q. Yu, Org. Lett. 2019, 21, 540;
- 4lH.-J. Xu, Y.-S. Kang, H. Shi, P. Zhang, Y.-K. Chen, B. Zhang, Z.-Q. Liu, J. Zhao, W.-Y. Sun, J.-Q. Yu, Y. Lu, J. Am. Chem. Soc. 2019, 141, 76;
- 4mJ. Xu, J. Chen, F. Gao, S. Xie, X. Xu, Z. Jin, J.-Q. Yu, J. Am. Chem. Soc. 2019, 141, 1903.
- 5
- 5aM. E. Welsch, S. A. Snyder, B. R. Stockwell, Curr. Opin. Chem. Biol. 2010, 14, 347;
- 5bY.-B. Wang, B. Tan, Acc. Chem. Res. 2018, 51, 534.
- 6
- 6aC. W. Liskey, X. Liao, J. F. Hartwig, J. Am. Chem. Soc. 2010, 132, 11389;
- 6bP. Anbarasan, T. Schareina, M. Beller, Chem. Soc. Rev. 2011, 40, 5049.
- 7
- 7aG.-J. Cheng, Y.-F. Yang, P. Liu, P. Chen, T.-Y. Sun, G. Li, X. Zhang, K. N. Houk, J.-Q. Yu, Y.-D. Wu, J. Am. Chem. Soc. 2014, 136, 894;
- 7bY.-F. Yang, G.-J. Cheng, P. Liu, D. Leow, T. Y. Sun, P. Chen, X. Zhang, J.-Q. Yu, Y.-D. Wu, K. N. Houk, J. Am. Chem. Soc. 2014, 136, 344;
- 7cL. Fang, T. G. Saint-Denis, B. L. H. Taylor, S. Ahlquist, K. Hong, S. Liu, L. Han, K. N. Houk, J.-Q. Yu, J. Am. Chem. Soc. 2017, 139, 10702;
- 7dY.-F. Yang, X. Hong, J.-Q. Yu, K. N. Houk, Acc. Chem. Res. 2017, 50, 2853.
- 8For examples of pyridine- or quinolone-ligand-promoted C−H activation, see:
- 8aJ. He, S. Li, Y. Deng, H. Fu, B. N. Laforteza, J. E. Spangler, A. Homs, J.-Q. Yu, Science 2014, 343, 1216;
- 8bS. Li, G. Chen, C.-G. Feng, W. Gong, J.-Q. Yu, J. Am. Chem. Soc. 2014, 136, 5267;
- 8cS. Li, R.-Y. Zhu, K.-J. Xiao, J.-Q. Yu, Angew. Chem. Int. Ed. 2016, 55, 4317; Angew. Chem. 2016, 128, 4389;
- 8dH. Park, N. Chekshin, P.-X. Shen, J.-Q. Yu, ACS Catal. 2018, 8, 9292.
- 9
- 9aP.-X. Shen, L. Hu, Q. Shao, K. Hong, J.-Q. Yu, J. Am. Chem. Soc. 2018, 140, 6545;
- 9bZ. Zhuang, C.-B. Yu, G. Chen, Q.-F. Wu, Y. Hsiao, C. L. Joe, J. X. Qiao, M. A. Poss, J.-Q. Yu, J. Am. Chem. Soc. 2018, 140, 10363.
- 10For examples of 2-pyridone-ligand-promoted C−H activation, see:
- 10aP. Wang, M. E. Farmer, X. Huo, P. Jain, P.-X. Shen, M. Ishoey, J. E. Bradner, S. R. Wisniewski, M. D. Eastgate, J.-Q. Yu, J. Am. Chem. Soc. 2016, 138, 9269;
- 10bP. Wang, P. Verma, G. Xia, J. Shi, J. X. Qiao, S. Tao, P. T. W. Cheng, M. A. Poss, M. E. Farmer, K.-S. Yeung, J.-Q. Yu, Nature 2017, 551, 489;
- 10cY.-Q. Chen, Z. Wang, Y. Wu, S. R. Wisniewski, J. X. Qiao, W. R. Ewing, M. D. Eastgate, J.-Q. Yu, J. Am. Chem. Soc. 2018, 140, 17884;
- 10dR.-Y. Zhu, Z.-Q. Li, H. S. Park, C. H. Senanayake, J.-Q. Yu, J. Am. Chem. Soc. 2018, 140, 3564.
- 11T. Mavromoustakos, G. Agelis, S. Durdagi, Expert Opin. Ther. Pat. 2013, 23, 1483.
- 12E. M. Simmons, J. F. Hartwig, Angew. Chem. Int. Ed. 2012, 51, 3066; Angew. Chem. 2012, 124, 3120.
- 13M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian 09; Gaussian Inc.: Wallingford, CT, 2009.
- 14
- 14aA. D. Becke, J. Chem. Phys. 1993, 98, 5648;
- 14bC. Lee, W. Yang, R. G. Parr, Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785;
- 14cA. D. Becke, J. Chem. Phys. 1993, 98, 1372;
- 14dP. J. Stephens, F. J. Devlin, C. F. Chabalowski, M. J. Frisch, J. Phys. Chem. 1994, 98, 11623.
- 15
- 15aR. Ditchfield, W. J. Hehre, J. A. Pople, J. Chem. Phys. 1971, 54, 724;
- 15bW. J. Hehre, R. Ditchfield, J. A. Pople, J. Chem. Phys. 1972, 56, 2257.
- 16
- 16aP. J. Hay, W. R. Wadt, J. Chem. Phys. 1985, 82, 299;
- 16bL. E. Roy, P. J. Hay, R. L. Martin, J. Chem. Theory Comput. 2008, 4, 1029.
- 17Y. Zhao, D. G. Truhlar, Theor. Chem. Acc. 2008, 120, 215.
- 18A. V. Marenich, C. J. Cramer, D. G. Truhlar, J. Phys. Chem. B 2009, 113, 6378.
- 19
- 19aM. Dolg, U. Wedig, H. Stoll, H. Preuss, J. Chem. Phys. 1987, 86, 866;
- 19bD. Andrae, U. Häussermann, M. Dolg, H. Stoll, H. Preuss, Theor. Chim. Acta 1990, 77, 123.
- 20C. Y. Legault, CYLview, 1.0b; Universitéde Sherbrooke, 2009 (http://www.cylview.org).