Rhodium-Catalyzed Enantioselective [4+2] Cycloadditions of Vinylcarbenes with Dienes
Bowen Zhang
Emory University, Chemistry, 1515 Dickey Drive, Atlanta, GA, 30024 USA
Search for more papers by this authorCorresponding Author
Huw M. L. Davies
Emory University, Chemistry, 1515 Dickey Drive, Atlanta, GA, 30024 USA
Search for more papers by this authorBowen Zhang
Emory University, Chemistry, 1515 Dickey Drive, Atlanta, GA, 30024 USA
Search for more papers by this authorCorresponding Author
Huw M. L. Davies
Emory University, Chemistry, 1515 Dickey Drive, Atlanta, GA, 30024 USA
Search for more papers by this authorGraphical Abstract
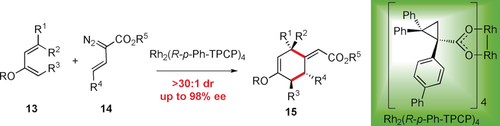
Selective [4+2] cycloaddition: A mechanistically unusual approach for a highly enantioselective [4+2] cycloaddition between rhodium-stabilized vinylcarbenes and siloxydienes was developed. The reaction is initiated by attack of the diene at the vinylogous position of the vinylcarbene and the outcome is dependent of the reacting conformation of the vinylcarbene intermediate.
Abstract
The reaction of 2-siloxycyclo-1,3-dienes with E-vinyldiazoacetates in the presence of the bulky chiral dirhodium tetracarboxylate catalyst, Rh2(R-p-PhTPCP)4 results in an enantioselective [4+2] cycloaddition, in which three new stereogenic centers are formed. The [4+2] cycloadducts are generated as single diastereomers with high enantiocontrol (95–98 % ee). When the diene contains an additional stereogenic center, effective kinetic resolution can be achieved.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201914354-sup-0001-misc_information.pdf10.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1H. M. L. Davies, D. M. Clarke, K. S. Thuy, Tetrahedron Lett. 1985, 26, 5659.
- 2H. M. L. Davies, E. G. Antoulinakis, Org. React. 2001, 57, 1–326.
- 3H. M. L. Davies, D. G. Stafford, B. D. Doan, J. H. Houser, J. Am. Chem. Soc. 1998, 120, 3326.
- 4H. M. L. Davies, P. Pelphrey, Org. React. 2011, 75, 75–212.
- 5H. M. L. Davies, Y. J. Lian, Acc. Chem. Res. 2012, 45, 923.
- 6J. R. Manning, H. M. L. Davies, J. Am. Chem. Soc. 2008, 130, 8602.
- 7H. M. L. Davies, E. Saikali, T. J. Clark, E. H. Chee, Tetrahedron Lett. 1990, 31, 6299.
- 8H. M. L. Davies, B. Hu, E. Saikali, P. R. Bruzinski, J. Org. Chem. 1994, 59, 4535.
- 9Reviews:
- 9aQ.-Q. Cheng, Y. Deng, L. Lankelma, M. P. Doyle, Chem. Soc. Rev. 2017, 46, 5425;
- 9bX. Xu, M. P. Doyle, Acc. Chem. Res. 2014, 47, 1396.
- 10Representative examples:
- 10aD. Valette, Y. J. Lian, J. P. Haydek, K. I. Hardcastle, H. M. L. Davies, Angew. Chem. Int. Ed. 2012, 51, 8636; Angew. Chem. 2012, 124, 8764;
- 10bA. G. Smith, H. M. L. Davies, J. Am. Chem. Soc. 2012, 134, 18241;
- 10cY. Deng, M. V. Yglesias, H. Arman, M. P. Doyle, Angew. Chem. Int. Ed. 2016, 55, 10108; Angew. Chem. 2016, 128, 10262;
- 10dX. Wang, X. Xu, P. Y. Zavalij, M. P. Doyle, J. Am. Chem. Soc. 2011, 133, 16402;
- 10eY. Qian, X. F. Xu, X. C. Wang, P. J. Zavalij, W. H. Hu, Angew. Chem. Int. Ed. 2012, 51, 5900; Angew. Chem. 2012, 124, 6002;
- 10fX. C. Wang, Q. M. Abrahams, P. J. Zavalij, M. P. Doyle, Angew. Chem. Int. Ed. 2012, 51, 5907; Angew. Chem. 2012, 124, 6009;
- 10gX. Xu, P. Y. Zavalij, M. P. Doyle, Angew. Chem. Int. Ed. 2012, 51, 9829; Angew. Chem. 2012, 124, 9967;
- 10hX. F. Xu, P. Y. Zavalij, M. P. Doyle, Angew. Chem. Int. Ed. 2013, 52, 12664; Angew. Chem. 2013, 125, 12896;
- 10iX. Xu, P. Y. Zavalij, M. P. Doyle, Chem. Commun. 2013, 49, 10287;
- 10jY. Qian, P. Y. Zavalij, W. Hu, M. P. Doyle, Org. Lett. 2013, 15, 1564;
- 10kX. Xu, P. Y. Zavalij, M. P. Doyle, J. Am. Chem. Soc. 2013, 135, 12439;
- 10lX. Xu, P. Y. Zavalij, W. Hu, M. P. Doyle, J. Org. Chem. 2013, 78, 1583;
- 10mX. Xu, J. S. Leszczynski, S. M. Mason, P. Y. Zavalij, M. P. Doyle, Chem. Commun. 2014, 50, 2462;
- 10nC. Jing, Q.-Q. Cheng, Y. Deng, H. Arman, M. P. Doyle, Org. Lett. 2016, 18, 4550;
- 10oQ.-Q. Cheng, J. Yedoyan, H. Arman, M. P. Doyle, J. Am. Chem. Soc. 2016, 138, 44;
- 10pA. S. Shved, A. A. Tabolin, R. A. Novikov, Y. V. Nelyubina, V. P. Timofeev, S. L. Ioffe, Eur. J. Org. Chem. 2016, 5569;
- 10qP. E. Guzmán, Y. Lian, H. M. L. Davies, Angew. Chem. Int. Ed. 2014, 53, 13083; Angew. Chem. 2014, 126, 13299;
- 10rY. Deng, L. A. Massey, P. Y. Zavalij, M. P. Doyle, Angew. Chem. Int. Ed. 2017, 56, 7479; Angew. Chem. 2017, 129, 7587.
- 11
- 11aY. J. Lian, H. M. L. Davies, Org. Lett. 2010, 12, 924;
- 11bY. J. Lian, H. M. L. Davies, Org. Lett. 2012, 14, 1934;
- 11cC. Qin, H. M. L. Davies, J. Am. Chem. Soc. 2013, 135, 14516.
- 12
- 12aX. Yang, Y. S. Yang, R. J. Rees, Q. Yang, Z. Y. Tian, Y. Xue, J. Org. Chem. 2016, 81, 8082;
- 12bL. L. Ma, W. Wang, G. C. Wang, RSC Adv. 2016, 6, 53839.
- 13D. M. Guptill, H. M. L. Davies, J. Am. Chem. Soc. 2014, 136, 17718.
- 14C. G. Espino, K. W. Fiori, M. Kim, J. Du Bois, J. Am. Chem. Soc. 2004, 126, 15378.
- 15The relative and absolute stereochemistry in the formation of 22 is tentatively assigned by analogy to the previous [4+3] cycloaddition studies (see ref. [3]), and the observation that the Rh2(S-DOSP)4 catalyst gives the opposite enantiomer to Rh2(R-p-PhTPCP)4 during the cyclopropanation stage, as reported in: S. Negretti, C. M. Cohen, J. J. Chang, D. M. Guptil, H. M. L. Davies, Tetrahedron 2015, 71, 7415.
- 16The crystal structure has been deposited at the Cambridge Crystallographic Data Centre, and the deposition number CCDC 1575130 has been allocated.
- 17The absolute configuration of compound 43 has not been unambiguously determined but is tentatively assigned by analogy to the X-Ray determined absolute configuration observed for the cycloaddition products.