Affecting an Ultra-High Work Function of Silver
Dr. Jin He
Institute of Chemistry and The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, 9190401 Israel
Search for more papers by this authorDr. Jeff Armstrong
ISIS Facility, Rutherford Appleton Laboratory, Harwell Oxford, Didcot, Oxfordshire, OX11 0QX UK
Search for more papers by this authorPeixi Cong
Department of Chemistry, University College of London, Gordon Street, London, WC1H 0AJ UK
Research Complex at Harwell, Rutherford Appleton Laboratory, Harwell Oxford, Didcot, Oxfordshire, OX11 0FA UK
Search for more papers by this authorBarak Menagen
Institute of Chemistry and The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, 9190401 Israel
Search for more papers by this authorLior Igaher
Institute of Chemistry and The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, 9190401 Israel
Search for more papers by this authorProf. Dr. Andrew M. Beale
Department of Chemistry, University College of London, Gordon Street, London, WC1H 0AJ UK
Research Complex at Harwell, Rutherford Appleton Laboratory, Harwell Oxford, Didcot, Oxfordshire, OX11 0FA UK
Search for more papers by this authorProf. Dr. Lioz Etgar
Institute of Chemistry and The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, 9190401 Israel
Search for more papers by this authorCorresponding Author
Prof. Dr. David Avnir
Institute of Chemistry and The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, 9190401 Israel
Search for more papers by this authorDr. Jin He
Institute of Chemistry and The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, 9190401 Israel
Search for more papers by this authorDr. Jeff Armstrong
ISIS Facility, Rutherford Appleton Laboratory, Harwell Oxford, Didcot, Oxfordshire, OX11 0QX UK
Search for more papers by this authorPeixi Cong
Department of Chemistry, University College of London, Gordon Street, London, WC1H 0AJ UK
Research Complex at Harwell, Rutherford Appleton Laboratory, Harwell Oxford, Didcot, Oxfordshire, OX11 0FA UK
Search for more papers by this authorBarak Menagen
Institute of Chemistry and The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, 9190401 Israel
Search for more papers by this authorLior Igaher
Institute of Chemistry and The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, 9190401 Israel
Search for more papers by this authorProf. Dr. Andrew M. Beale
Department of Chemistry, University College of London, Gordon Street, London, WC1H 0AJ UK
Research Complex at Harwell, Rutherford Appleton Laboratory, Harwell Oxford, Didcot, Oxfordshire, OX11 0FA UK
Search for more papers by this authorProf. Dr. Lioz Etgar
Institute of Chemistry and The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, 9190401 Israel
Search for more papers by this authorCorresponding Author
Prof. Dr. David Avnir
Institute of Chemistry and The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, 9190401 Israel
Search for more papers by this authorGraphical Abstract
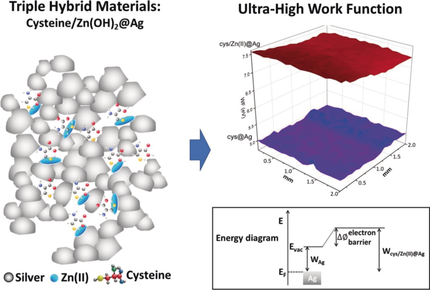
An ultra-high increase in the work function (WF) of silver, from 4.26 to 7.42 eV, by 3D dual-entrapment of the WF modifying components l-cysteine and Zn(OH)2 within the metal is reported. The WF enhancement mechanism is based on directly affecting the charge transfer ability of the metal, separately by cysteine and hydrolyzed zinc(II), and synergistically by the combination of the two, through the known Zn-cysteine finger redox trap effect.
Abstract
An ultra-high increase in the WF of silver, from 4.26 to 7.42 eV, that is, an increase of up to circa 3.1 eV is reported. This is the highest WF increase on record for metals and is supported by recent computational studies which predict the potential ability to affect an increase of the WF of metals by more than 4 eV. We achieved the ultra-high increase by a new approach: Rather than using the common method of 2D adsorption of polar molecules layers on the metal surface, WF modifying components, l-cysteine and Zn(OH)2, were incorporated within the metal, resulting in a 3D architecture. Detailed material characterization by a large array of analytical methods was carried out, the combination of which points to a WF enhancement mechanism which is based on directly affecting the charge transfer ability of the metal separately by cysteine and hydrolyzed zinc(II), and synergistically by the combination of the two through the known Zn-cysteine finger redox trap effect.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201912293-sup-0001-misc_information.pdf898.8 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1N. D. Lang, W. Kohn, Phys. Rev. B 1971, 3, 1215.
- 2I. H. Campbell, S. Rubin, T. A. Zawodzinski, J. D. Kress, R. L. Martin, D. L. Smith, N. N. Barashkov, J. P. Ferraris, Phys. Rev. B 1996, 54, R 14321.
- 3H. Zhang, J. Tang, Q. Zhang, G. Zhao, G. Yang, J. Zhang, O. Zhou, L. Qin, Adv. Mater. 2006, 18, 87–91.
- 4J. P. Barnak, R. S. Chau, C. Liang, US Patent US7022559B2, 2006.
- 5B. de Boer, A. Hadipour, M. M. Mandoc, T. van Woudenbergh, P. W. M. Blom, Adv. Mater. 2005, 17, 621–625.
- 6M. L. Sushko, A. L. Shluger, Adv. Mater. 2009, 21, 1111–1114.
- 7V. B. Engelkes, J. M. Beebe, C. D. Frisbie, J. Am. Chem. Soc. 2004, 126, 14287–14296.
- 8O. T. Hofmann, D. A. Egger, E. Zojer, Nano Lett. 2010, 10, 4369–4374.
- 9P. D. Taylor, D. A. Osborne, S. A. Tawfik, T. Morishita, M. J. S. Spencer, Phys. Chem. Chem. Phys. 2019, 21, 7165–7173.
- 10O. T. Hofmann, H. Glowatzki, C. Bürker, G. M. Rangger, B. Bürker, J. Niederhausen, T. Hosokai, I. Salzmann, R.-P. Blum, R. Rieger, J. Phys. Chem. C 2017, 121, 24657–24668.
- 11J. He, L. Iagher, L. Etgar, D. Avnir, Chem. Commun. 2018, 54, 7203–7206.
- 12D. Avnir, Adv. Mater. 2018, 30, 1706804.
- 13D. Avnir, Acc. Chem. Res. 2014, 47, 579–592.
- 14N. Ralbag, M. Mann-Lahav, E. S. Davydova, U. Ash, R. Galed, M. Handl, R. Hiesgen, E. Magliocca, W. Mustain, J. He, P. Cong, A. M. Beale, G. S. Grader, D. Avnir, D. R. Dekel, Matter 2019, 1, 959–975.
- 15T. S. Bauer, B. Menagen, D. Avnir, Z. Hayouka, Sci. Rep. 2019, 9, 1–8.
- 16B. Menagen, D. Avnir, ACS Biomater. Sci. Eng. 2019, 5, 2355–2364.
- 17L. Shapiro, D. Avnir, ChemCatChem 2017, 9, 816–823.
- 18N. Ralbag, I. Felner, D. Avnir, Phys. Rev. B 2019, 99, 64411.
- 19R. Ben-Knaz, D. Avnir, Biomaterials 2009, 30, 1263–1267.
- 20P. Li, Z. P. Xu, M. A. Hampton, D. T. Vu, L. Huang, V. Rudolph, A. V. Nguyen, J. Phys. Chem. C 2012, 116, 10325–10332.
- 21V. Shkirskiy, P. Keil, H. Hintze-Bruening, F. Leroux, F. Brisset, K. Ogle, P. Volovitch, Corros. Sci. 2015, 100, 101–112.
- 22N. Pace, E. Weerapana, Biomolecules 2014, 4, 419–434.
- 23F. D. Urnov, J. C. Miller, Y.-L. Lee, C. M. Beausejour, J. M. Rock, S. Augustus, A. C. Jamieson, M. H. Porteus, P. D. Gregory, M. C. Holmes, Nature 2005, 435, 646.
- 24M. Bibikova, K. Beumer, J. K. Trautman, D. Carroll, Science 2003, 300, 764.
- 25W. Maret, Antioxid. Redox Signaling 2006, 8, 1419–1441.
- 26K.-D. Kröncke, L.-O. Klotz, Antioxid. Redox Signaling 2009, 11, 1015–1027.
- 27B. O. Leung, F. Jalilehvand, V. Mah, M. Parvez, Q. Wu, Inorg. Chem. 2013, 52, 4593–4602.
- 28N. H. Helal, W. A. Badawy, Electrochim. Acta 2011, 56, 6581–6587.
- 29M. B. Radovanović, M. B. Petrović, A. T. Simonović, S. M. Milić, M. M. Antonijević, Environ. Sci. Pollut. Res. 2013, 20, 4370–4381.
- 30D. Kesavan, M. Gopiraman, N. Sulochana, Chem. Sci. Rev. Lett. 2012, 1, 1–8.
- 31S. Fischer, A. C. Papageorgiou, M. Marschall, J. Reichert, K. Diller, F. Klappenberger, F. Allegretti, A. Nefedov, C. Wöll, J. V. Barth, J. Phys. Chem. C 2012, 116, 20356–20362.
- 32Q. Chen, N. V. Richardson, Nat. Mater. 2003, 2, 324.
- 33T. N. Ramesh, T. L. Madhu, Int. J. Inorg. Chem. 2015, 0, 536470.
- 34A. K. Yadav, S. M. Haque, S. Tripathi, D. Shukla, M. A. Ahmed, D. M. Phase, S. Bandyopadhyay, S. N. Jha, D. Bhattacharyya, RSC Adv. 2016, 6, 74982–74990.
- 35H. Behar-Levy, G. E. Shter, G. S. Grader, D. Avnir, Chem. Mater. 2004, 16, 3197–3202.
- 36G. Nesher, M. Aylien, G. Sandaki, D. Avnir, G. Marom, Adv. Funct. Mater. 2009, 19, 1293–1298.
- 37V. A. Yablokov, Y. A. Vasina, I. A. Zelyaev, S. V. Mitrofanova, Russ. J. Gen. Chem. 2009, 79, 1141.
- 38S. Foley, M. Enescu, Vib. Spectrosc. 2007, 44, 256–265.
- 39X. Wu, N. H. Bishopric, D. J. Discher, B. J. Murphy, K. A. Webster, Mol. Cell. Biol. 1996, 16, 1035–1046.
- 40S. F. Parker, A. J. Ramirez-Cuesta, L. Daemen, Spectrochim. Acta Part A 2018, 190, 518–523.
- 41S. F. Parker, F. Fernandez-Alonso, A. J. Ramirez-Cuesta, J. Tomkinson, S. Rudic, R. S. Pinna, G. Gorini, J. Fernández Castañon, J. Phys. Conf. Ser. 2014, 554, 012003.
- 42K. M. Ismail, Electrochim. Acta 2007, 52, 7811–7819.
- 43R. W. Zehner, B. F. Parsons, R. P. Hsung, L. R. Sita, Langmuir 1999, 15, 1121–1127.
- 44S. Prada, U. Martinez, G. Pacchioni, Phys. Rev. B 2008, 78, 235423.
- 45S. Choi, D.-H. Lee, S. G. Louie, J. Clarke, Phys. Rev. Lett. 2009, 103, 197001.
- 46C. Cen, S. Thiel, G. Hammerl, C. W. Schneider, K. E. Andersen, C. S. Hellberg, J. Mannhart, J. Levy, Nat. Mater. 2008, 7, 298.
- 47M. Sterrer, T. Risse, U. M. Pozzoni, L. Giordano, M. Heyde, H.-P. Rust, G. Pacchioni, H.-J. Freund, Phys. Rev. Lett. 2007, 98, 96107.
- 48S. Lu, Z. Qin, Q. Guo, G. Cao, Appl. Surf. Sci. 2017, 392, 849–853.
- 49L. Giordano, F. Cinquini, G. Pacchioni, Phys. Rev. B 2006, 73, 45414.
- 50E. Zojer, T. C. Taucher, O. T. Hofmann, Adv. Mater. Interfaces 2019, 6, 1900581.
- 51C. Hu, D. Liu, Y. Xiao, L. Dai, Prog. Nat. Sci. Mater. Int. 2018, 28, 121–132.