Solid-State and Gas-Phase Structures and Energetic Properties of the Dangerous Methyl and Fluoromethyl Nitrates
Dedicated to Professor Hubert Schmidbaur on the occasion of his 85th birthday
Graphical Abstract
Abstract
An improved synthesis of the simplest nitric acid ester, methyl nitrate, and a new synthesis of fluoromethyl nitrate use the metathesis of the corresponding iodomethanes with silver nitrate. Both compounds were identified by spectroscopy and the structures determined for in situ grown crystals by X-ray diffraction as well as in the gas phase by electron diffraction. Fluorination leads to structures with shorter C−O and N−O bonds, has an energetically destabilizing effect and increases friction sensitivity, but decreases detonation performance.
Potential energetic materials are typically screened for density, performance, stability, and sensitivity towards friction and impact.1 In general, high density contributes to high performance.2 The influence of fluorine substituents on energetic materials is well documented, but almost nothing is known about the important parameter sensitivity towards impact and friction. These sensitivities were frequently rationalized with numerous and short inter- and intramolecular open-shell interactions.3 Understanding the mutual interactions between atoms and functional groups is crucial to develop safe-to-handle energetic materials. Small and simple, yet highly energetic molecules are particularly suitable for exploring the effect of H/F exchange on the sensitivities due to the limited number of intermolecular interactions.4 These molecules are often highly sensitive to impact and friction. The challenge is to find suitable molecules whose sensitivities can be determined by conventional methods and to compare them with non- and polyfluorinated derivatives, as was recently demonstrated for perchloric acid esters.5
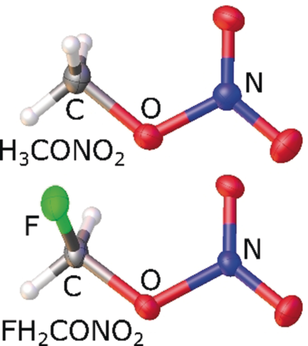
Fluoromethyl nitrate (FCH2ONO2, FMN) is one of three fluorine-containing derivatives of methyl nitrate, CH3ONO2 (MN),6 besides F2CHONO2 (DFMN)8 and F3CONO2 (TFMN).9 Organic nitrates are important energetic compounds widely used in military and aviation industries, but so far FMN (and also DFMN) has been studied only by ab initio calculations.7, 10 In contrast, TFMN (m.p. −163 °C, b.p. −18 °C) is isolable, but unstable even at low temperatures.8, 9
Methyl nitrate (MN, m.p. −82 °C, b.p. 65 °C), so-called Schießwasser (German for shooting water), was used as early as 1420, though then not recognized as this material.11a, 11b Mysterious accidents were attributed to MN between 1933 and 1955 and again in the 1980s.11c–11g Despite its unflattering reputation, various synthetic protocols, properties, and applications have been reported.6, 10, 12 The first structure elucidation of this toxic and consciousness-altering substance dates back to 1937 with theoretical and initial gas-phase electron diffraction (GED) studies.13 Solid-state structures determined by single-crystal X-ray diffraction of MN and FMN have not been available so far, but could serve to compute electrostatic potentials, often used to explain changes in sensitivity, and for comparison with quantum-mechanical results.2, 14
The original synthesis of MN, the nitration of methanol with nitric acid, cannot be adopted for FMN. This would require starting from fluoromethanol, which is known to be unstable and readily decomposes into HF and formaldehyde under ambient conditions.6, 15 However, the adaptation of an ethyl nitrate synthesis via silver-catalyzed heterolysis16 is successful: iodomethane or fluoroiodomethane is reacted with silver nitrate (Scheme 1). MN and FMN (m.p. −91 °C, b.p. 58 °C) were both isolated as strong-smelling, colorless, volatile liquids. They cause severe headache upon exposure.

Synthesis of MN and FMN.
Identification and characterization is possible by NMR spectroscopy. In contrast to the 1H NMR resonance of the methyl group in MN (4.10 ppm), the methylene group of FMN gives rise to a doublet at 5.98 ppm (2JF,H=52.0 Hz); the downfield shift is due to the strong electron-withdrawing effect of fluorine. FMN shows a triplet at −155.9 ppm in its 19F NMR spectrum, and a doublet of triplets at 99.1 ppm (1JF,C=228.8, 1JC,H=182.4 Hz) in its 13C NMR spectrum.
The 15N NMR signal of FMN at −52.4 ppm is a triplet of doublets (3JN,H=6.7, 3JF,N=1.7 Hz; Figure 1); that is, substitution of MN (−39.4 ppm, quartet 3JN,H=3.9 Hz) by one fluorine atom leads to an upfield shift. The 17O resonance (obtained using highly concentrated solutions, Figure 1) of the FCH2O unit in FMN at 363 ppm is shifted downfield relative to the methoxy resonance in MN at 310 ppm. In contrast, the NO2 resonance at 446 ppm remains unaffected upon H/F exchange. The chemical shifts of both FMN and MN recorded in CD3CN solution are similar to those of neat ethyl nitrate (340, 470 ppm).17
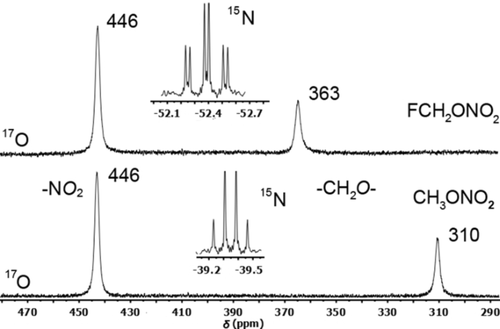
15N and 17O NMR spectra of FMN (top) and MN (bottom) in CD3CN (26 °C).
Selected vibrations of the IR and Raman spectra of MN and FMN are listed in in Table 1. The IR stretching vibrations of the NO2 group for FMN are found at 1670 cm−1 (νasNO2) and 1291 cm−1 (νsNO2). Compared to MN, these vibrational modes are shifted to higher wavenumbers due to the electronegative F substituent. The lower values of the νNO stretching vibration of FMN (IR, 811 cm−1) indicates a weaker N−O(CH2F) bond upon F/H substitution. The experimental data differ in part from earlier calculated data, likely due to the liquid state.7
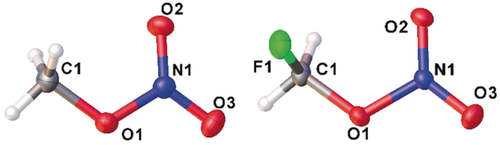
Molecular structures of MN (left) and FMN (right) in the solid state. Ellipsoids are set at the 50 % probability level. Numbering holds for the gas-phase structures as well.
|
MN |
FMN |
||||||
|---|---|---|---|---|---|---|---|---|
|
IR |
Raman |
IR |
Raman |
||||
|
expt. |
calcd. |
expt. |
calcd. |
expt. |
calcd. |
expt. |
calcd. |
νasNO2 |
1622 (s) |
1714 (s) |
1636 (w) |
1714 (w) |
1670 (s) |
1767 (s) |
1689 (w) |
1767 (w) |
νsNO2 |
1281 (s) |
1324 (s) |
1285 (m) |
1324 (w) |
1291 (s) |
1340 (m) |
1296 (m) |
1340 (w) |
νCF |
– |
– |
– |
– |
1047 (m) |
1032 (w) |
1049 (w) |
1032 (w) |
νCO |
989 (s) |
1015 (m) |
991 (m) |
1015 (m) |
996 (s) |
1023 (s) |
1005 (w) |
1023 (w) |
νNO |
854 (s) |
862 (s) |
860 (m) |
862 (m) |
811 (s) |
824 (s) |
822 (m) |
824 (m) |
δNO2 |
652 (m) |
661 (m) |
664 (w) |
661 (m) |
654 (m) |
647 (w) |
660 (w) |
647 (m) |
MN and FMN were structurally characterized in the gas phase by electron diffraction (GED, Table 2) and in the case of MN also by combining GED data with rotational constants (Table 3; details given in the Supporting Information). Figure 3 shows the radial distribution curves for the GED experiments. While MN adopts Cs symmetry with one of the hydrogen atoms in antiperiplanar position to the nitrogen atom, the fluorine atom in FMN resides gauche relative to the planar NO2 unit (ϕ(F1C1O1N1)=74.7(8)°). Fluorination has severe effects on the structure parameters: in FMN the C−O1 and N−O2/O3 distances are shortened by 0.04 Å (MN 1.425(3), FMN 1.385(3) Å) and 0.01 Å (MN 1.205(1), 1.198(1) Å, FMN 1.190(2), 1.185(1) Å), respectively. In variance, the O1−N distance in FMN is about 0.05 Å longer than in MN (MN 1.403(2), FMN 1.454(2) Å). This is likely due to negative hyperconjugation of the oxygen lone pairs into the antibonding orbitals of the C−F and NO bonds. The C-O1-N angle in FMN (115.3°) is 2° greater than in MN.
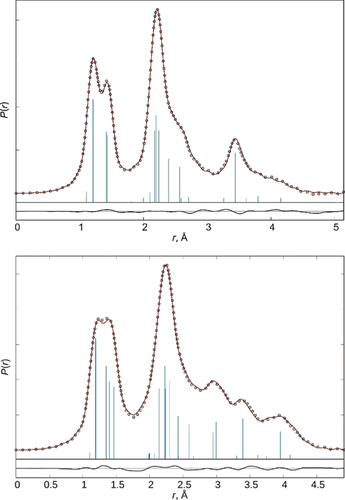
Experimental (circles) and model (line) radial distribution functions of MN (top) and FMN (bottom). The line below is the difference curve. Vertical bars indicate interatomic distances in the molecule.
Parameter |
MN |
FMN |
||
|---|---|---|---|---|
|
XRD |
GED+RotC |
XRD |
GED |
C-O |
1.451(1) |
1.425(3) |
1.412(2) |
1.385(3) |
O1-N |
1.388(1) |
1.403(2) |
1.433(2) |
1.454(2) |
N-O2 |
1.204(1) |
1.205(1) |
1.208(2) |
1.190(2) |
N-O3 |
1.212(1) |
1.198(1) |
1.200(2) |
1.185(1) |
C-F |
|
|
1.379(2) |
1.336(2) |
C-O-N |
113.3(1) |
113.6(3) |
113.3(1) |
115.3(2) |
O1-N-O2 |
118.5(1) |
116.3(3) |
118.1(1) |
115.1(3) |
O1-N-O3 |
112.9(1) |
112.3(2) |
111.9(1) |
111.9(11) |
O2-N-O3 |
128.6(1) |
131.4(4) |
130.1(1) |
133.0(13) |
F-C-O-N |
|
|
79.7(1) |
74.7(8) |
Parameter |
MP2(full)/cc-pwCVTZ |
GED+RotC[a] |
wGED[b] [%] |
|---|---|---|---|
C1−O1 |
1.426 |
1.425(3) |
48 |
O1−N1 |
1.407 |
1.403(2) |
40 |
N1−O2 |
1.207 |
1.205(1) |
64 |
N1−O3 |
1.201 |
1.198(1) |
64 |
average C−H |
1.084 |
1.080(5) |
49 |
C1-O1-N1 |
112.2 |
113.6(3) |
14 |
O1N1O2 |
117.1 |
116.3(3) |
17 |
O1N1O3 |
112.6 |
112.3(2) |
7 |
O2N1O3 |
130.3 |
131.4(4) |
8 |
wRMSD[c] [MHz] |
15.9 |
2.7 |
|
R-factor[d] [%] |
7.0[e] |
4.8 |
- [a] Values correspond to equilibrium structure. In parentheses are total standard deviations obtained from Monte Carlo simulations as described earlier.19 [b] Contribution of GED data to refined value, estimated according to the W2 method.20 [c] Weighted root-mean-square deviation of model rotational constants from experimental. [d] Disagreement factor between model and experimental electron diffraction intensities. [e] Model refined against GED data with geometrical parameters fixed at ab initio values.
The solid-state structures of both nitrates were determined by X-ray diffraction of in situ grown crystals (Figure 2). An unexpected small crystal of oxonium nitrate dihydrate obtained during crystallization of MN was also structurally characterized (details in the Supporting Information). MN crystallizes in the space group Pbca and FMN in Cc. Both contain one molecule per asymmetric unit.18 In both molecules, the carbon, nitrogen, and oxygen atoms are almost coplanar; the root mean square deviation is 0.001 Å. The Cs symmetry of MN is broken by the torsion angles of the methyl group ϕ(NOCH): 175.6(7)°, 65.9(7)°, and 60.0(7)°. FMN adopts a gauche conformation with a torsion angle ϕ(NOCF) of 79.7(1)°; ϕ(NOCH) angles are 169(2)° and 40(2)°. As in the gas phase, structural changes upon fluorination result in a shorter C1−O1 bond (MN 1.451(1) Å; FMN 1.412(2) Å), a longer O1−N1 bond (MN 1.388(1) Å; FMN 1.433(2) Å) and slightly shorter N1−O2/O3 bonds (MN 1.204(1)/ 1.212(1) Å, FMN 1.208(2)/ 1.200(2) Å).
Solid MN and FMN contain N⋅⋅⋅O and N⋅⋅⋅F contacts shorten than or close to the van der Waals distances (3.07/ 3.02 Å) (Figure 4). Two independent N⋅⋅⋅O contacts in MN have lengths of 3.094(1) (N1⋅⋅⋅O3′) and 3.042(1) Å (N1⋅⋅⋅O2′′) and a corresponding angle O3′⋅⋅⋅N1⋅⋅⋅O2′′ of 171.9(1)°. Comparable contacts in FMN are significantly shorter at 2.928(2) Å (N1⋅⋅⋅O2′) and 2.895(2) Å (N1⋅⋅⋅F1′′) and the angle O2′⋅⋅⋅N1⋅⋅⋅F1′′ at 168.1(1)° is narrower. Thus, both crystal structures feature pseudo-trigonal-bipyramidally coordinated nitrogen atoms with intermolecular contacts in axial position.
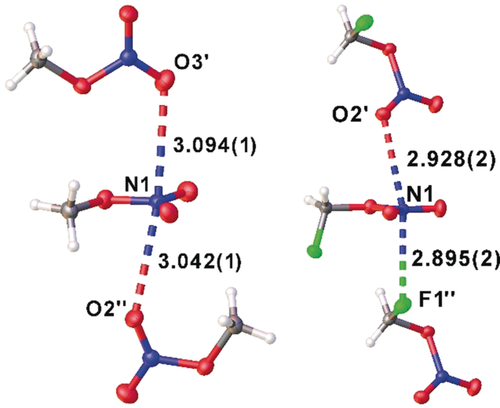
Molecular assembly of methyl nitrate and fluoromethyl nitrate in the solid state. Symmetry operations generating equivalent positions for MN: (−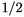 +x, y, 3/2−z) for (′) and (
+x, y, 3/2−z) for (′) and (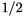 +x,
+x, 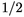 −y, 1−z) for (′′), for FMN: (+x, 1−y,
−y, 1−z) for (′′), for FMN: (+x, 1−y,  +z) for (′) and (−
+z) for (′) and (− +x,
+x,  −y, −
−y, − +z) for (′′).
+z) for (′′).
The influence of H/F substitution on the energetic properties was determined and results for MN11e, 11f, 21 and FMN are listed in Table 4. The sensitivity of MN and FMN towards friction and impact was determined experimentally according to the standards of the German Federal Institute for Material Research and Testing (BAM).22 The two nitrates show the same sensitivity to impact of 0.2 J. However, the friction sensitivity of FMN is significantly higher than that of MN. Thus, the UN recommendations on transport of dangerous goods require FMN to be classified as very sensitive towards impact and as sensitive towards friction.23
|
MN |
FMN |
|---|---|---|
formula |
CH3NO3 |
CH2FNO3 |
M [g mol−1] |
77.04 |
95.03 |
IS[a] [J] |
0.2 |
0.2 |
FS[b] [N] |
353 |
108 |
N[c] [%] |
18.18 |
14.74 |
N+O+F[d] [%] |
80.48 |
85.24 |
ΩCO[e] [%] |
10.4 |
25.3 |
ΩCO2[e] [%] |
−10.4 |
8.4 |
Tmelt[f] [°C] |
−83.0 |
−90 |
Tboil[g] [°C] |
65.0 |
58.0 |
ρ100K[h] [g cm−3] (XRD) |
1.579 |
1.838 |
ρ293K[i] [g cm−3] |
1.21 |
1.28 |
ΔHf0[j] [kJ mol−1] |
−162.3 |
−361.7 |
|
|
|
EXPLO5 V 6.03 |
|
|
ΔUf0 [k] [kJ kg−1] |
−6021 |
−4450 |
TC-J[l] [K] |
4151 |
3827 |
PC-J[m] [GPa] |
14.2 |
12.3 |
Vdet[n] [ms−1] |
6653 |
6133 |
Vo[o] [dm3 kg−1] |
923.7 |
836.8 |
- [a] Impact sensitivity (BAM drop-hammer, method 1 of 6). [b] Friction sensitivity (BAM friction tester, method 1 of 6). [c] Nitrogen content. [d] Combined nitrogen, oxygen, and fluorine content. [e] Absolute oxygen balance assuming the formation of CO or CO2 and HF. [f] Melting point. [g] Boiling point determined by the Siwoloboff method. [h] Density determined by X-ray diffraction at 100 K. [i] Experimentally determined density at 293 K. [j] Heat of formation calculated at the CBS-4M level of theory. [k] Detonation energy. [l] Detonation temperature. [m] Detonation pressure. [n] Detonation velocity. [o] Volume of detonation gases at standard temperature and pressure conditions.
In contrast to impact or shock sensitivity, friction sensitivity does not usually attract the attention of theoreticians, but there seems to be a correlation between friction sensitivity and electrostatic potential (ESP).24 The ESP of FMN differs significantly from that of MN, which may be related to the significantly greater impact sensitivity (Figure 5).2 For FMN the positive region (blue) is larger and the positive potential (max. +100 kJ mol−1) is greater than for MN. The maximum negative potentials at the NO2 unit (−44 kJ mol−1) and the F atom (−52 kJ mol−1) in FMN are much less negative than in MN. This is in contrast to the situation in MN, which has a more strongly negative (max. −84 kJ mol−1) than positive region. This and the fact that there is a higher positive potential in the molecular center indicate FMN to be more friction sensitive.2, 14b The weaker negative potential (maximum: −44/−52 vs.−84 kJ mol−1) is probably the main reason for the increased friction sensitivity.24 A destabilizing effect of fluorine substitution was already evoked to explain the high instability of trifluoromethyl nitrate (TFMN).9 Initial results on methylene dinitrate CH2(ONO2)2,25 prepared in analogy to FMN, confirm this increased instability (see the Supporting Information).26 Consequently, it is not surprising that attempts to synthesize the multiply fluorine/nitrate-substituted FCH(ONO2)2 from FCHI2 were not successful. An immediate decomposition into N2O5 (hydrolyzing to HNO3) and “FCHO” was proven by NMR spectroscopy.27
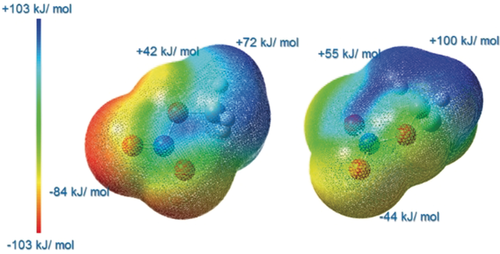
ESP of MN (left) and FMN (right), isovalue=0.02.
Quantum-chemical calculations were carried out for MN and FMN. Heats of formation were computed using optimized structures28 and are considerably more negative for FMN than for MN (Table 4). Based on these values and the corresponding densities at ambient temperature, detonation parameters of MN and FMN were calculated using the EXPLO5 V6.03 code30 (Table 4). Calculations at the Chapman–Jouguet (C-J) point applied a stationary detonation model with a modified Becker–Kistiakowski–Wilson state equation. The C-J point was located using the first derivative of the Hugoniot curve of the system.31 The calculated detonation parameters are comparable with those of glycerine trinitrate (ΔUf0 −6099 kJ kg−1, TC-J 4316 K, PC-J 23.7 GPa, Vdet 7850 ms−1, Vo 781 dm3 kg−1). The heat of detonation, detonation pressure, velocity, and temperature of glycerine trinitrate are all higher than those of MN and FMN, but the gas volumes released from MN and FMN are smaller.
In essence we have synthesized and characterized fluoromethyl nitrate for comparison with methyl nitrate in order to learn about the effect of fluorine substitution on various structural and energetic parameters. We find shorter C−O and N−O bonds and a wider C-O-N angle in the fluorinated species. Fluorine substitution has a destabilizing effect: it increases friction sensitivity but decreases detonation performance.
Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation): core facility GED@BI (project no. 324757882) and a structure elucidation grant for MN and FMN (project no. 416982996). We thank Ludwig-Maximilian University for financial support, F-Select GmbH for a gift of fluoroiodomethane, and A. Harter for participating in this project. We gratefully acknowledge computing time provided by the Paderborn Center for Parallel Computing (PC2).
Conflict of interest
The authors declare no conflict of interest.