Emerging Bottom-Up Strategies for the Synthesis of Graphene Nanoribbons and Related Structures
Anthony Jolly
Department of Chemistry and Centre de Recherche sur les Matériaux Avancés, Université Laval, 1045 Ave de la Médecine, Quebec, QC, G1V 0A6 Canada
Search for more papers by this authorDandan Miao
Department of Chemistry and Centre de Recherche sur les Matériaux Avancés, Université Laval, 1045 Ave de la Médecine, Quebec, QC, G1V 0A6 Canada
Search for more papers by this authorMaxime Daigle
Department of Chemistry and Centre de Recherche sur les Matériaux Avancés, Université Laval, 1045 Ave de la Médecine, Quebec, QC, G1V 0A6 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Jean-François Morin
Department of Chemistry and Centre de Recherche sur les Matériaux Avancés, Université Laval, 1045 Ave de la Médecine, Quebec, QC, G1V 0A6 Canada
Search for more papers by this authorAnthony Jolly
Department of Chemistry and Centre de Recherche sur les Matériaux Avancés, Université Laval, 1045 Ave de la Médecine, Quebec, QC, G1V 0A6 Canada
Search for more papers by this authorDandan Miao
Department of Chemistry and Centre de Recherche sur les Matériaux Avancés, Université Laval, 1045 Ave de la Médecine, Quebec, QC, G1V 0A6 Canada
Search for more papers by this authorMaxime Daigle
Department of Chemistry and Centre de Recherche sur les Matériaux Avancés, Université Laval, 1045 Ave de la Médecine, Quebec, QC, G1V 0A6 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Jean-François Morin
Department of Chemistry and Centre de Recherche sur les Matériaux Avancés, Université Laval, 1045 Ave de la Médecine, Quebec, QC, G1V 0A6 Canada
Search for more papers by this authorGraphical Abstract
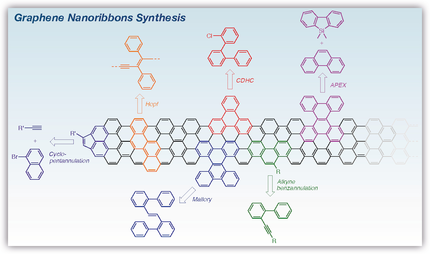
To prepare high-quality, defect-free graphene nanoribbons (GNRs), cycloaromatization reactions need to be very efficient, proceed without side reaction and mild enough to accommodate various functional groups. In this Minireview, the latest approaches for the synthesis of GNRs and related structures, including alkyne benzannulation, photochemical cyclodehydrohalogenation, Mallory and Pd- and Ni-catalyzed reactions are presented.
Abstract
The solution-phase synthesis is one of the most promising strategies for the preparation of well-defined graphene nanoribbons (GNRs) in large scale. To prepare high quality, defect-free GNRs, cycloaromatization reactions need to be very efficient, proceed without side reaction and mild enough to accommodate the presence of various functional groups. In this Minireview, we present the latest synthetic approaches for the synthesis of GNRs and related structures, including alkyne benzannulation, photochemical cyclodehydrohalogenation, Mallory and Pd- and Ni-catalyzed reactions.
Conflict of interest
The authors declare no conflict of interest.
References
- 1K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos, A. A. Firsov, Science 2004, 306, 666–669.
- 2A. K. Geim, K. S. Novoselov, Nat. Mater. 2007, 6, 183–91.
- 3“Graphene Flagship Annual Report 2018 From the Director,” can be found under https://graphene-flagship.eu/SiteCollectionDocuments/Admin/Annualreport/GrapheneFlagshipAnnualReport2018.pdf, 2018.
- 4S. Das, D. Pandey, J. Thomas, T. Roy, Adv. Mater. 2019, 31, 1802722.
- 5R. Raccichini, A. Varzi, S. Passerini, B. Scrosati, Nat. Mater. 2015, 14, 271–279.
- 6H. A. Hafez, S. Kovalev, J. C. Deinert, Z. Mics, B. Green, N. Awari, M. Chen, S. Germanskiy, U. Lehnert, J. Teichert, et al., Nature 2018, 561, 507–511.
- 7K. Choi, A. Droudian, R. M. Wyss, K.-P. Schlichting, H. G. Park, Sci. Adv. 2018, 4, eaau 0476.
- 8Y. Liang, X. Liang, Z. Zhang, W. Li, X. Huo, L. Peng, Nanoscale 2015, 7, 10954–10962.
- 9G. X. Ni, A. S. McLeod, Z. Sun, L. Wang, L. Xiong, K. W. Post, S. S. Sunku, B. Y. Jiang, J. Hone, C. R. Dean, et al., Nature 2018, 557, 530–533.
- 10E. Singh, M. Meyyappan, H. S. Nalwa, ACS Appl. Mater. Interfaces 2017, 9, 34544–34586.
- 11D. G. Papageorgiou, I. A. Kinloch, R. J. Young, Prog. Mater. Sci. 2017, 90, 75–127.
- 12K. I. Bolotin, K. J. Sikes, Z. Jiang, M. Klima, G. Fudenberg, J. Hone, P. Kim, H. L. Stormer, Solid State Commun. 2008, 146, 351–355.
- 13S. J. Zhang, S. S. Lin, X. Q. Li, X. Y. Liu, H. A. Wu, W. L. Xu, P. Wang, Z. Q. Wu, H. K. Zhong, Z. J. Xu, Nanoscale 2016, 8, 226–232.
- 14R. Martinazzo, S. Casolo, G. F. Tantardini, Phys. Rev. B 2010, 81, 1–8.
- 15H. Zhang, E. Bekyarova, J. W. Huang, Z. Zhao, W. Bao, F. Wang, R. C. Haddon, C. N. Lau, Nano Lett. 2011, 11, 4047–4051.
- 16S. Dutta, S. K. Pati, J. Mater. Chem. 2010, 20, 8207–8223.
- 17D. V. Kosynkin, A. L. Higginbotham, A. Sinitskii, J. R. Lomeda, A. Dimiev, B. K. Price, J. M. Tour, Nature 2009, 458, 872–876.
- 18M. Y. Han, B. Özyilmaz, Y. Zhang, P. Kim, Phys. Rev. Lett. 2007, 98, 1–4.
- 19L. Zhang, H. Dai, S. Lee, X. Wang, X. Li, Science 2008, 319, 1229–1232.
- 20L. Ma, J. Wang, F. Ding, ChemPhysChem 2013, 14, 47–54.
- 21J. Cai, P. Ruffieux, R. Jaafar, M. Bieri, T. Braun, S. Blankenburg, M. Muoth, A. P. Seitsonen, M. Saleh, X. Feng, et al., Nature 2010, 466, 470–473.
- 22H. Sakaguchi, Y. Kawagoe, Y. Hirano, T. Iruka, M. Yano, T. Nakae, Adv. Mater. 2014, 26, 4134–4138.
- 23H. Zhang, H. Lin, K. Sun, L. Chen, Y. Zagranyarski, N. Aghdassi, S. Duhm, Q. Li, D. Zhong, Y. Li, et al., J. Am. Chem. Soc. 2015, 137, 4022–4025.
- 24M. Di Giovannantonio, O. Deniz, J. I. Urgel, R. Widmer, T. Dienel, S. Stolz, C. Sánchez-Sánchez, M. Muntwiler, T. Dumslaff, R. Berger, et al., ACS Nano 2018, 12, 74–81.
- 25C. Bronner, R. A. Durr, D. J. Rizzo, Y. L. Lee, T. Marangoni, A. M. Kalayjian, H. Rodriguez, W. Zhao, S. G. Louie, F. R. Fischer, et al., ACS Nano 2018, 12, 2193–2200.
- 26A. Narita, Z. Chen, Q. Chen, K. Müllen, Chem. Sci. 2019, 10, 964–975.
- 27I. A. Verzhbitskiy, A. Narita, C. Casiraghi, W. Frederickx, X. Feng, K. S. Mali, M. Bonn, K. Müllen, M. R. Hansen, S. A. Jensen, et al., ACS Nano 2014, 8, 11622–11630.
- 28L. Chen, Y. Hernandez, X. Feng, K. Müllen, Angew. Chem. Int. Ed. 2012, 51, 7640–7654; Angew. Chem. 2012, 124, 7758–7773.
- 29L. Dössel, L. Gherghel, X. Feng, K. Müllen, Angew. Chem. Int. Ed. 2011, 50, 2540–2543; Angew. Chem. 2011, 123, 2588–2591.
- 30M. G. Schwab, A. Narita, S. Osella, Y. Hu, A. Maghsoumi, A. Mavrinsky, W. Pisula, C. Castiglioni, M. Tommasini, D. Beljonne, et al., Chem. Asian J. 2015, 10, 2134–2138.
- 31A. Narita, X.-Y. Wang, X. Feng, K. Müllen, Chem. Soc. Rev. 2015, 44, 6616–6643.
- 32Y. Hu, P. Xie, M. De Corato, A. Ruini, S. Zhao, F. Meggendorfer, L. A. Straasø, L. Rondin, P. Simon, J. Li, et al., J. Am. Chem. Soc. 2018, 140, 7803–7809.
- 33M. G. Schwab, A. Narita, Y. Hernandez, T. Balandina, K. S. Mali, S. De Feyter, X. Feng, K. Müllen, J. Am. Chem. Soc. 2012, 134, 18169–18172.
- 34J. Liu, B.-W. Li, Y.-Z. Tan, A. Giannakopoulos, C. Sanchez-Sanchez, D. Beljonne, P. Ruffieux, R. Fasel, X. Feng, K. Müllen, J. Am. Chem. Soc. 2015, 137, 6097–6103.
- 35X. Yang, X. Dou, A. Rouhanipour, L. Zhi, H. J. Räder, K. Müllen, J. Am. Chem. Soc. 2008, 130, 4216–4217.
- 36G. Li, K. Y. Yoon, X. Zhong, J. Wang, R. Zhang, J. R. Guest, J. Wen, X. Y. Zhu, G. Dong, Nat. Commun. 2018, 9, 1–9.
- 37M. Grzybowski, K. Skonieczny, H. Butenschön, D. T. Gryko, Angew. Chem. Int. Ed. 2013, 52, 9900–9930; Angew. Chem. 2013, 125, 10084–10115.
- 38F. Cataldo, Polyynes. Synthesis Properties and Applications, CRC Press, New York, 2006.
- 39G. Wegner, Z. Naturforsch. B 1969, 24, 824–832.
- 40G. Wegner, Makromol. Chem. 1972, 154, 35–48.
- 41O. M. Behr, G. Eglinton, A. R. Galbraith, R. A. Raphael, J. Chem. Soc. 1960, 3614.
- 42Q. Zhou, P. J. Carroll, T. M. Swager, J. Org. Chem. 1994, 59, 1294–1301.
- 43L. Luo, D. Resch, C. Wilhelm, C. N. Young, G. P. Halada, R. J. Gambino, C. P. Grey, N. S. Goroff, J. Am. Chem. Soc. 2011, 133, 19274–19277.
- 44S. Rondeau-Gagné, J. F. Morin, Chem. Soc. Rev. 2014, 43, 85–98.
- 45J. F. Morin, Synlett 2013, 24, 2032–2044.
- 46E. T. Chernick, R. R. Tykwinski, J. Phys. Org. Chem. 2013, 26, 742–749.
- 47J. R. Néabo, C. Vigier-Carrière, S. Rondeau-Gagné, J. F. Morin, Chem. Commun. 2012, 48, 10144–10146.
- 48L. Ding, S. V. Olesik, Nano Lett. 2004, 4, 2271–2276.
- 49S. Rondeau-Gagné, J. Roméo-Néabo, M. Desroches, J. Larouche, J. Brisson, J.-F. Morin, J. Am. Chem. Soc. 2013, 135, 110–113.
- 50K. Maeda, L. Hong, T. Nishihara, Y. Nakanishi, Y. Miyauchi, R. Kitaura, N. Ousaka, E. Yashima, H. Ito, K. Itami, J. Am. Chem. Soc. 2016, 138, 11001–11008.
- 51J. R. Néabo, S. Rondeau-Gagné, C. Vigier-Carrieìre, J. F. Morin, Langmuir 2013, 29, 3446–3452.
- 52S. Schrettl, C. Stefaniu, C. Schwieger, G. Pasche, E. Oveisi, Y. Fontana, A. F. I. Morral, J. Reguera, R. Petraglia, C. Corminboeuf, et al., Nat. Chem. 2014, 6, 468–476.
- 53I. Levesque, J. R. Néabo, S. Rondeau-Gagné, C. Vigier-Carrière, M. Daigle, J. F. Morin, Chem. Sci. 2014, 5, 831–836.
- 54R. S. Jordan, Y. Wang, R. D. McCurdy, M. T. Yeung, K. L. Marsh, S. I. Khan, R. B. Kaner, Y. Rubin, Chem 2016, 1, 78–90.
- 55R. S. Jordan, Y. L. Li, C.-W. Lin, R. D. McCurdy, J. B. Lin, J. L. Brosmer, K. L. Marsh, S. I. Khan, K. N. Houk, R. B. Kaner, et al., J. Am. Chem. Soc. 2017, 139, 15878–15890.
- 56L. Yang, C. H. Park, Y. W. Son, M. L. Cohen, S. G. Louie, Phys. Rev. Lett. 2007, 99, 186801.
- 57M. B. Goldfinger, T. M. Swager, J. Am. Chem. Soc. 1994, 116, 7895–7896.
- 58C. Li, C. Wang, H. Liao, R. Chaudhuri, R. Liu, J. Org. Chem. 2007, 72, 9203–9207.
- 59U. Rohr, C. Kohl, K. Müllen, A. Van De Craats, J. Warman, J. Mater. Chem. 2001, 11, 1789–1799.
- 60M. B. Goldfinger, K. B. Crawford, T. M. Swager, J. Am. Chem. Soc. 1997, 119, 4578–4593.
- 61A. Senese, W. Chalifoux, Molecules 2018, 24, 118.
- 62W. Yang, A. Lucotti, M. Tommasini, W. A. Chalifoux, J. Am. Chem. Soc. 2016, 138, 9137–9144.
- 63W. Yang, J. H. S. K. Monteiro, A. de Bettencourt-Dias, V. J. Catalano, W. A. Chalifoux, Angew. Chem. Int. Ed. 2016, 55, 10427–10430; Angew. Chem. 2016, 128, 10583–10586.
- 64W. Yang, R. R. Kazemi, N. Karunathilake, V. J. Catalano, M. A. Alpuche-Aviles, W. A. Chalifoux, Org. Chem. Front. 2018, 5, 2288–2295.
- 65W. Yang, G. Longhi, S. Abbate, A. Lucotti, M. Tommasini, C. Villani, V. J. Catalano, A. O. Lykhin, S. A. Varganov, W. A. Chalifoux, J. Am. Chem. Soc. 2017, 139, 13102–13109.
- 66W. Yang, W. A. Chalifoux, Synlett 2017, 28, 625–632.
- 67W. Yang, R. Bam, V. J. Catalano, W. A. Chalifoux, Angew. Chem. Int. Ed. 2018, 57, 14773–14777; Angew. Chem. 2018, 130, 14989–14993.
- 68N. Asao, T. Nogami, S. Lee, Y. Yamamoto, J. Am. Chem. Soc. 2003, 125, 10921–10925.
- 69H. Arslan, J. D. Saathoff, D. N. Bunck, P. Clancy, W. R. Dichtel, Angew. Chem. Int. Ed. 2012, 51, 12051–12054; Angew. Chem. 2012, 124, 12217–12220.
- 70H. Arslan, F. J. Uribe-Romo, B. J. Smith, W. R. Dichtel, Chem. Sci. 2013, 4, 3973–3978.
- 71D. Lehnherr, C. Chen, Z. Pedramrazi, C. R. Deblase, J. M. Alzola, I. Keresztes, E. B. Lobkovsky, M. F. Crommie, W. R. Dichtel, Chem. Sci. 2016, 7, 6357–6364.
- 72B. Itin, H. Arslan, C. R. Crick, Y.-L. Loo, J. Gao, W. R. Dichtel, P. Clancy, J. D. Saathoff, F. J. Uribe-Romo, S. J. Hein, ACS Nano 2016, 10, 4847–4856.
- 73C. Lütke Eversloh, Y. Avlasevich, C. Li, K. Müllen, Chem. Eur. J. 2011, 17, 12756–12762.
- 74J. D. Wood, J. L. Jellison, A. D. Finke, L. Wang, K. N. Plunkett, J. Am. Chem. Soc. 2012, 134, 15783–15789.
- 75C. H. Lee, K. N. Plunkett, Org. Lett. 2013, 15, 1202–1205.
- 76G. C. Kulkarni, J. L. Morales-Cruz, W. A. Hussain, I. J. Garvey, K. N. Plunkett, Synlett 2018, 29, 2572–2576.
- 77S. R. Bheemireddy, P. C. Ubaldo, P. W. Rose, A. D. Finke, J. Zhuang, L. Wang, K. N. Plunkett, Angew. Chem. Int. Ed. 2015, 54, 15762–15766; Angew. Chem. 2015, 127, 15988–15992.
- 78S. R. Bheemireddy, M. P. Hautzinger, T. Li, B. Lee, K. N. Plunkett, J. Am. Chem. Soc. 2017, 139, 5801–5807.
- 79F. B. Mallory, C. W. Mallory, in Org. React., 2007, pp. 1–456.
- 80L. Liu, B. Yang, T. J. Katz, M. K. Poindexter, J. Org. Chem. 1991, 56, 3769–3775.
- 81K. N. Plunkett, K. Godula, C. Nuckolls, N. Tremblay, A. C. Whalley, S. Xiao, Org. Lett. 2009, 11, 2225–2228.
- 82C.-Y. Chiu, B. Kim, A. A. Gorodetsky, W. Sattler, S. Wei, A. Sattler, M. Steigerwald, C. Nuckolls, Chem. Sci. 2011, 2, 1480–1486.
- 83X. Zhang, J. Li, H. Qu, C. Chi, J. Wu, Org. Lett. 2010, 12, 2008–2011.
- 84M. Ball, Y. Zhong, Y. Wu, C. Schenck, F. Ng, M. Steigerwald, S. Xiao, C. Nuckolls, Acc. Chem. Res. 2015, 48, 267–276.
- 85S. Xiao, S. J. Kang, Y. Wu, S. Ahn, J. B. Kim, Y.-L. Loo, T. Siegrist, M. L. Steigerwald, H. Li, C. Nuckolls, Chem. Sci. 2013, 4, 2018.
- 86Z. Yuan, Y. Xiao, X. Qian, Chem. Commun. 2010, 46, 2772–2774.
- 87Z. Zhang, T. Lei, Q. Yan, J. Pei, D. Zhao, Chem. Commun. 2013, 49, 2882.
- 88P. E. Hartnett, H. S. S. Ramakrishna Matte, N. D. Eastham, N. E. Jackson, Y. Wu, L. X. Chen, M. A. Ratner, R. P. H. Chang, M. C. Hersam, M. R. Wasielewski, et al., Chem. Sci. 2016, 7, 3543–3555.
- 89X. Li, K. Wu, L. Zheng, Y. Deng, S. Tan, H. Chen, Dye. Pigment. 2019, 168, 59–67.
- 90H. Zhylitskaya, M. Stépień, Org. Chem. Front. 2018, 5, 2395–2414.
- 91S. R. Peurifoy, J. C. Russell, T. J. Sisto, Y. Yang, X. Roy, C. Nuckolls, J. Am. Chem. Soc. 2018, 140, 10960–10964.
- 92N. J. Schuster, R. Hernández Sánchez, D. Bukharina, N. A. Kotov, N. Berova, F. Ng, M. L. Steigerwald, C. Nuckolls, J. Am. Chem. Soc. 2018, 140, 6235–6239.
- 93G. Liu, T. Koch, Y. Li, N. L. Doltsinis, Z. Wang, Angew. Chem. Int. Ed. 2019, 58, 178–183; Angew. Chem. 2019, 131, 184–189.
- 94N. J. Schuster, D. W. Paley, S. Jockusch, F. Ng, M. L. Steigerwald, C. Nuckolls, Angew. Chem. Int. Ed. 2016, 55, 13519–13523; Angew. Chem. 2016, 128, 13717–13721.
- 95L. Chen, C. Li, K. Müllen, J. Mater. Chem. C 2014, 2, 1938–1956.
- 96Y. Zhong, B. Kumar, S. Oh, M. T. Trinh, Y. Wu, K. Elbert, P. Li, X. Zhu, S. Xiao, F. Ng, et al., J. Am. Chem. Soc. 2014, 136, 8122–8130.
- 97T. J. Sisto, Y. Zhong, B. Zhang, M. T. Trinh, K. Miyata, X. Zhong, X. Y. Zhu, M. L. Steigerwald, F. Ng, C. Nuckolls, J. Am. Chem. Soc. 2017, 139, 5648–5651.
- 98Y. Zhong, T. J. Sisto, B. Zhang, K. Miyata, X. Y. Zhu, M. L. Steigerwald, F. Ng, C. Nuckolls, J. Am. Chem. Soc. 2017, 139, 5644–5647.
- 99W. A. Henderson, A. Zweig, J. Am. Chem. Soc. 1967, 89, 6778–6779.
- 100R. S. Davidson, J. W. Goodin, G. Kemp, The Photochemistry of Aryl Halides and Related Compounds, Elsevier, Amsterdam, 1984.
10.1016/S0065-3160(08)60149-5 Google Scholar
- 101J. Grimshaw, A. P. De Silva, Chem. Soc. Rev. 1981, 10, 181–203.
- 102M. Daigle, A. Picard-Lafond, E. Soligo, J. F. Morin, Angew. Chem. Int. Ed. 2016, 55, 2042–2047; Angew. Chem. 2016, 128, 2082–2087.
- 103M. Daigle, D. Miao, A. Lucotti, M. Tommasini, J. F. Morin, Angew. Chem. Int. Ed. 2017, 56, 6213–6217; Angew. Chem. 2017, 129, 6309–6313.
- 104D. Miao, M. Daigle, A. Lucotti, J. Boismenu-Lavoie, M. Tommasini, J. F. Morin, Angew. Chem. Int. Ed. 2018, 57, 3588–3592; Angew. Chem. 2018, 130, 3650–3654.
- 105D. Miao, C. Aumaitre, J. F. Morin, J. Mater. Chem. C 2019, 7, 3015–3024.
- 106M. Daigle, J. F. Morin, Macromolecules 2017, 50, 9257–9264.
- 107F. Schlütter, T. Nishiuchi, V. Enkelmann, K. Müllen, Angew. Chem. Int. Ed. 2014, 53, 1538–1542; Angew. Chem. 2014, 126, 1564–1568.
- 108D. Jänsch, I. Ivanov, Y. Zagranyarski, I. Duznovic, M. Baumgarten, D. Turchinovich, C. Li, M. Bonn, K. Müllen, Chem. Eur. J. 2017, 23, 4870–4875.
- 109W. Zeng, Q. Qi, J. Wu, Eur. J. Org. Chem. 2018, 7–17.
- 110H. Ito, Y. Segawa, K. Murakami, K. Itami, J. Am. Chem. Soc. 2019, 141, 3–10.
- 111H. Ito, K. Ozaki, K. Itami, Angew. Chem. Int. Ed. 2017, 56, 11144–11164; Angew. Chem. 2017, 129, 11296–11317.
- 112K. Ozaki, K. Kawasumi, M. Shibata, H. Ito, K. Itami, Nat. Commun. 2015, 6, 1–8.
- 113W. Matsuoka, H. Ito, K. Itami, Angew. Chem. Int. Ed. 2017, 56, 12224–12228; Angew. Chem. 2017, 129, 12392–12396.
- 114Y. Koga, T. Kaneda, Y. Saito, K. Murakami, K. Itami, Science 2018, 359, 435–439.
- 115C. Zhu, D. Wang, D. Wang, Y. Zhao, W. Y. Sun, Z. Shi, Angew. Chem. Int. Ed. 2018, 57, 8848–8853; Angew. Chem. 2018, 130, 8986–8991.