Aggregation-Induced Electrochemiluminescence of Carboranyl Carbazoles in Aqueous Media
Xing Wei
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Nanjing University, Nanjing, 210023 China
These authors contributed equally to this work.
Search for more papers by this authorMeng-Jiao Zhu
State Key Laboratory of Analytical Chemistry for Life Science, Nanjing University, Nanjing, 210023 China
These authors contributed equally to this work.
Search for more papers by this authorZhe Cheng
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorMengjeu Lee
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hong Yan
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Changsheng Lu
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jing-Juan Xu
State Key Laboratory of Analytical Chemistry for Life Science, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorXing Wei
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Nanjing University, Nanjing, 210023 China
These authors contributed equally to this work.
Search for more papers by this authorMeng-Jiao Zhu
State Key Laboratory of Analytical Chemistry for Life Science, Nanjing University, Nanjing, 210023 China
These authors contributed equally to this work.
Search for more papers by this authorZhe Cheng
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorMengjeu Lee
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Hong Yan
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Changsheng Lu
State Key Laboratory of Coordination Chemistry, Jiangsu Key Laboratory of Advanced Organic Materials, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jing-Juan Xu
State Key Laboratory of Analytical Chemistry for Life Science, Nanjing University, Nanjing, 210023 China
Search for more papers by this authorGraphical Abstract
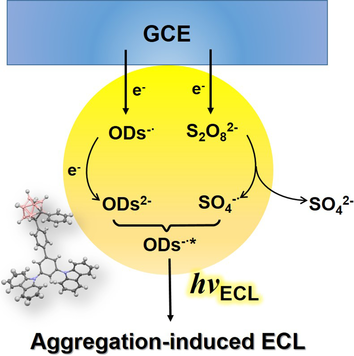
Carborane-based AIEgens: The reductive–oxidative aggregation-induced electrochemiluminescence (AIECL) of carboranyl carbazoles in air-saturated aqueous media was investigated. Mechanistic studies indicate that the carboranyl motif plays vital role in the high ECL intensity and stability of the aggregates.
Abstract
The aggregation-induced electrochemiluminescence (AIECL) of carboranyl carbazoles in aqueous media was investigated for the first time. Quantum yields, morphologies, and particle sizes were observed to determine the electrochemiluminescence (ECL) performance of these aggregated organic dots (ODs). All compounds exhibit much higher ECL stability and intensity than the carborane-free compound, demonstrating the essential role of the carboranyl motif. Moreover, the results of cyclic voltammetry (CV) suggest that oxidation/reduction reactions take place at the carboranyl motif. The excited states of ODs were proposed to be generated by the mechanism of surface state transitions. More importantly, these compounds show a reductive–oxidative mechanism in contrast to other organic materials that show oxidative–reductive mechanisms. Our experiments and data have established the relation between AIE organic structures and ECL properties that has a strong potential for biological and diagnostic applications.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201900283-sup-0001-misc_information.pdf2.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. N. Grimes in Carboranes, Vol. 9, 2nd ed., Academic Press, New York, 2011, pp. 301–540;
- 1bN. S. Hosmane, R. Eagling in Handbook of Boron Science, Vol. 4, Stallion Press, Sharjah, 2019, pp. 156–161;
- 1cN. S. Hosmane, Boron science: new technologies and applications, CRC, Boca Raton, 2011, pp. 675–699.
10.1201/b11199-34 Google Scholar
- 2
- 2aM. Herberhold, H. Yan, W. Milius, B. Wrackmeyer, Angew. Chem. Int. Ed. 1999, 38, 3689–3691;
10.1002/(SICI)1521-3773(19991216)38:24<3689::AID-ANIE3689>3.0.CO;2-T CAS PubMed Web of Science® Google ScholarAngew. Chem. 1999, 111, 3888–3890;10.1002/(SICI)1521-3757(19991216)111:24<3888::AID-ANGE3888>3.0.CO;2-B Web of Science® Google Scholar
- 2bX. Zhang, H. Yan, Coord. Chem. Rev. 2019, 378, 466–482;
- 2cX. Zhang, H. Zheng, J. Li, F. Xu, J. Zhao, H. Yan, J. Am. Chem. Soc. 2017, 139, 14511–14517;
- 2dX. Zhang, H. Yan, Chem. Sci. 2018, 9, 3964–3969;
- 2eR. Zhang, L. Zhu, G. Liu, H. Dai, Z. Lu, J. Zhao, H. Yan, J. Am. Chem. Soc. 2012, 134, 10341–10344;
- 2fZ. Wang, H. Ye, Y. Li, Y. Li, H. Yan, J. Am. Chem. Soc. 2013, 135, 11289–11298;
- 2gD. Zhao, Z. Xie, Coord. Chem. Rev. 2016, 314, 14–33;
- 2hR. Cheng, Z. Qiu, Z. Xie, Nat. Commun. 2017, 8, 14827;
- 2iC. Tang, J. Zhang, J. Zhang, Z. Xie, J. Am. Chem. Soc. 2018, 140, 16423–16427;
- 2jR. Cheng, B. Li, J. Wu, J. Zhang, Z. Qiu, W. Tang, S.-L. You, Y. Tang, Z. Xie, J. Am. Chem. Soc. 2018, 140, 4508–4511;
- 2kF. Lin, J.-L. Yu, Y. Shen, S.-Q. Zhang, B. Spingler, J. Liu, X. Hong, S. Duttwyler, J. Am. Chem. Soc. 2018, 140, 13798–13807;
- 2lT.-T. Xu, K. Cao, J. Wu, C.-Y. Zhang, J. Yang, Inorg. Chem. 2018, 57, 2925–2932;
- 2mG. J. Planas, F. Teixidor, C. Viñas, Crystals 2016, 6, 50;
- 2nR. Núñez, M. Tarrés, A. Ferrer-Ugalde, F. F. de Biani, F. Teixidor, Chem. Rev. 2016, 116, 14307–14378;
- 2oS. M. Gao, N. S. Hosmane, Russ. Chem. Bull. 2014, 63, 788–810.
- 3
- 3aC. Bellomo, M. Chaari, J. Cabrera-González, M. Blangetti, C. Lombardi, A. Deagostino, C. Viñas, N. Gaztelumendi, C. Nogués, R. Nuñez, C. Prandi, Chem. Eur. J. 2018, 24, 15622–15630;
- 3bL. Zhu, W. Lv, S. Liu, H. Yan, Q. Zhao, W. Huang, Chem. Commun. 2013, 49, 10638–10640;
- 3cX. Li, Y. Yin, P. Gao, W. Li, H. Yan, C. Lu, Q. Zhao, Dalton Trans. 2017, 46, 13802–13810;
- 3dA. F. Armstrong, J. F. Valliant, Dalton Trans. 2007, 4240–4251.
- 4
- 4aM. Couto, M. F. García, C. Alamón, M. Cabrera, P. Cabral, A. Merlino, F. Teixidor, H. Cerecetto, C. Viñas, Chem. Eur. J. 2018, 24, 3122–3126;
- 4bC. Wu, L. Shi, Q. Li, H. Jiang, M. Selke, H. Yan, X. Wang, Nanomedicine: Nanotechnology, Biology and Medicine 2012, 8, 860–869;
- 4cR. Kuhnert, M.-B. Sárosi, S. George, P. Lönnecke, B. Hofmann, D. Steinhilber, S. Steinmann, R. Schneider-Stock, B. Murganić, S. Mijatović, D. Maksimović-Ivanić, E. Hey-Hawkins, ChemMedChem 2018, https://doi.org/10.1002/cmdc.201800651;
- 4dD. J. Worm, S. Els-Heindl, M. Kellert, R. Kuhnert, S. Saretz, J. Koebberling, B. Riedl, E. Hey-Hawkins, A. G. Beck-Sickinger, J. Pept. Sci. 2018, 24, e 3119;
- 4eM. Scholz, A. L. Blobaum, L. J. Marnett, E. Hey-Hawkins, Bioorg. Med. Chem. 2011, 19, 3242–3248.
- 5
- 5aA. N. Ay, H. Akar, A. Zaulet, C. Viňas, F. Teixidor, B. Zumreoglu-Karan, Dalton Trans. 2017, 46, 3303–3310;
- 5bM. P. Grzelczak, S. P. Danks, R. C. Klipp, D. Belic, A. Zaulet, C. Kunstmann-Olsen, D. F. Bradley, T. Tsukuda, C. Viñas, F. Teixidor, J. J. Abramson, M. Brust, ACS Nano 2017, 11, 12492–12499;
- 5cS. Janczak, A. Olejniczak, S. Balabańska, M. K. Chmielewski, M. Lupu, C. Viñas, Z. J. Lesnikowski, Chem. Eur. J. 2015, 21, 15118–15122.
- 6
- 6aG. Calabrese, A. Daou, E. Barbu, J. Tsibouklis, Drug Discovery Today 2018, 23, 63–75;
- 6bI. Takeuchi, K. Nomura, K. Makino, Colloids Surf. B 2017, 159, 360–365;
- 6cJ. F. Valliant, K. J. Guenther, A. S. King, P. Morel, P. Schaffer, O. O. Sogbein, K. A. Stephenson, Coord. Chem. Rev. 2002, 232, 173–230;
- 6dM. Scholz, E. Hey-Hawkins, Chem. Rev. 2011, 111, 7035–7062;
- 6eH. S. Ban, H. Nakamura, Chem. Rec. 2015, 15, 616–635;
- 6fH. Nakamura, Y. Yasui, H. S. Ban, J. Organomet. Chem. 2013, 747, 189–194.
- 7
- 7aC. Shi, H. Sun, X. Tang, W. Lv, H. Yan, Q. Zhao, J. Wang, W. Huang, Angew. Chem. Int. Ed. 2013, 52, 13434–13438; Angew. Chem. 2013, 125, 13676–13680;
- 7bX. Li, X. Tong, Y. Yin, H. Yan, C. Lu, W. Huang, Q. Zhao, Chem. Sci. 2017, 8, 5930–5940;
- 7cY. H. Lee, J. Park, J. Lee, S. U. Lee, M. H. Lee, J. Am. Chem. Soc. 2015, 137, 8018–8021;
- 7dJ. C. Axtell, K. O. Kirlikovali, P. I. Djurovich, D. Jung, V. T. Nguyen, B. Munekiyo, A. T. Royappa, A. L. Rheingold, A. M. Spokoyny, J. Am. Chem. Soc. 2016, 138, 15758–15765.
- 8D. Tu, P. Leong, S. Guo, H. Yan, C. Lu, Q. Zhao, Angew. Chem. Int. Ed. 2017, 56, 11370–11374; Angew. Chem. 2017, 129, 11528–11532.
- 9
- 9aH. Naito, K. Nishino, Y. Morisaki, K. Tanaka, Y. Chujo, Angew. Chem. Int. Ed. 2017, 56, 254–259; Angew. Chem. 2017, 129, 260–265;
- 9bJ. Li, C. Yang, X. Peng, Y. Chen, Q. Qi, X. Luo, W.-Y. Lai, W. Huang, J. Mater. Chem. C 2018, 6, 19–28;
- 9cY. Yin, X. Li, S. Yan, H. Yan, C. Lu, Chem. Asian J. 2018, 13, 3155–3159;
- 9dX. Li, Y. Yin, H. Yan, C. Lu, Chem. Asian J. 2017, 12, 2207–2210;
- 9eA. V. Marsh, N. J. Cheetham, M. Little, M. Dyson, A. J. P. White, P. Beavis, C. N. Warriner, A. C. Swain, P. N. Stavrinou, M. Heeney, Angew. Chem. Int. Ed. 2018, 57, 10640–10645; Angew. Chem. 2018, 130, 10800–10805.
- 10
- 10aM. Z. Shafikov, A. F. Suleymanova, R. Czerwieniec, H. Yersin, Inorg. Chem. 2017, 56, 13274–13285;
- 10bN. V. Nghia, S. Jana, S. Sujith, J. Y. Ryu, J. Lee, S. U. Lee, M. H. Lee, Angew. Chem. Int. Ed. 2018, 57, 12483–12488; Angew. Chem. 2018, 130, 12663–12668.
- 11R. Furue, T. Nishimoto, I. S. Park, J. Lee, T. Yasuda, Angew. Chem. Int. Ed. 2016, 55, 7171–7175; Angew. Chem. 2016, 128, 7287–7291.
- 12
- 12aJ. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu, B. Z. Tang, Chem. Commun. 2001, 1740–1741;
- 12bJ. Liang, G. Feng, R. T. K. Kwok, D. Ding, B. Tang, B. Liu, Sci. China Chem. 2016, 59, 53–61;
- 12cM. Gao, B. Z. Tang, ACS Sens. 2017, 2, 1382–1399.
- 13
- 13aJ. Qian, B. Z. Tang, Chem 2017, 3, 56–91;
- 13bJ. Mei, Y. Huang, H. Tian, ACS Appl. Mater. Interfaces 2018, 10, 12217–12261;
- 13cM. Gao, B. Z. Tang, Drug Discovery Today 2017, 22, 1288–1294;
- 13dX. Gu, R. T. K. Kwok, J. W. Y. Lam, B. Z. Tang, Biomaterials 2017, 146, 115–135;
- 13eD. Ding, K. Li, B. Liu, B. Z. Tang, Acc. Chem. Res. 2013, 46, 2441–2453.
- 14Y. Zhang, Y. Wang, J. Wang, X.-J. Liang, Mater. Horiz. 2018, 5, 799–812.
- 15
- 15aJ. Wang, L. Chen, J. Ye, Z. Li, H. Jiang, H. Yan, M. Y. Stogniy, I. B. Sivaev, V. I. Bregadze, X. Wang, Biomacromolecules 2017, 18, 1466–1472;
- 15bM. Białek-Pietras, A. B. Olejniczak, E. Paradowska, M. Studzińska, A. Jabłońska, Z. J. Leśnikowski, J. Organomet. Chem. 2018, 865, 166–172;
- 15cA. V. Efremenko, A. A. Ignatova, M. A. Grin, I. B. Sivaev, A. F. Mironov, V. I. Bregadze, A. V. Feofanov, Photochem. Photobiol. Sci. 2014, 13, 92–102;
- 15dA. M. Cioran, A. D. Musteti, F. Teixidor, Ž. Krpetić, I. A. Prior, Q. He, C. J. Kiely, M. Brust, C. Viñas, J. Am. Chem. Soc. 2012, 134, 212–221.
- 16
- 16aZ. Liu, W. Qi, G. Xu, Chem. Soc. Rev. 2015, 44, 3117–3142;
- 16bM. M. Richter, Chem. Rev. 2004, 104, 3003–3036.
- 17Y. Chen, S. Zhou, L. Li, J.-j. Zhu, Nano Today 2017, 12, 98–115.
- 18
- 18aR. Ishimatsu, S. Matsunami, T. Kasahara, J. Mizuno, T. Edura, C. Adachi, K. Nakano, T. Imato, Angew. Chem. Int. Ed. 2014, 53, 6993–6996; Angew. Chem. 2014, 126, 7113–7116;
- 18bF. Rizzo, F. Polo, G. Bottaro, S. Fantacci, S. Antonello, L. Armelao, S. Quici, F. Maran, J. Am. Chem. Soc. 2017, 139, 2060–2069;
- 18cH. Qi, J. J. Teesdale, R. C. Pupillo, J. Rosenthal, A. J. Bard, J. Am. Chem. Soc. 2013, 135, 13558–13566;
- 18dK. N. Swanick, S. Ladouceur, E. Zysman-Colman, Z. Ding, Angew. Chem. Int. Ed. 2012, 51, 11079–11082; Angew. Chem. 2012, 124, 11241–11244.
- 19J. E. Dick, C. Renault, B.-K. Kim, A. J. Bard, J. Am. Chem. Soc. 2014, 136, 13546–13549.
- 20
- 20aS. Zanarini, E. Rampazzo, S. Bonacchi, R. Juris, M. Marcaccio, M. Montalti, F. Paolucci, L. Prodi, J. Am. Chem. Soc. 2009, 131, 14208–14209;
- 20bH. Lee, J. Kim, ChemElectroChem 2017, 4, 1790–1796;
- 20cP. Chauhan, K. Chu, N. Yan, Z. Ding, J. Electroanal. Chem. 2016, 781, 181–189.
- 21
- 21aS. Carrara, A. Aliprandi, C. F. Hogan, L. De Cola, J. Am. Chem. Soc. 2017, 139, 14605–14610;
- 21bT.-B. Gao, J.-J. Zhang, R.-Q. Yan, D.-K. Cao, D. Jiang, D. Ye, Inorg. Chem. 2018, 57, 4310–4316.
- 22
- 22aZ. Wang, Y. Feng, N. Wang, Y. Cheng, Y. Quan, H. Ju, J. Phys. Chem. Lett. 2018, 9, 5296–5302;
- 22bF. Sun, Z. Wang, Y. Feng, Y. Cheng, H. Ju, Y. Quan, Biosens. Bioelectron. 2018, 100, 28–34.
- 23P. Wu, X. Hou, J.-J. Xu, H.-Y. Chen, Chem. Rev. 2014, 114, 11027–11059.
- 24
- 24aA. J. Stewart, K. Brown, L. Dennany, Anal. Chem. 2018, 90, 12944–12950;
- 24bB. Babamiri, A. Salimi, R. Hallaj, Biosens. Bioelectron. 2018, 117, 332–339;
- 24cY. N. Khonsari, S. Sun, Microchim. Acta 2018, 185, 430.
- 25
- 25aA. Jimeno-Romero, E. Bilbao, E. Valsami-Jones, M. P. Cajaraville, M. Soto, I. Marigómez, Ecotoxicol. Environ. Saf. 2019, 167, 288–300;
- 25bW. N. Missaoui, R. D. Arnold, B. S. Cummings, Chem.-Biol. Interact. 2018, 295, 1–12.
- 26
- 26aH. Uoyama, K. Goushi, K. Shizu, H. Nomura, C. Adachi, Nature 2012, 492, 234;
- 26bB. Wex, B. R. Kaafarani, J. Mater. Chem. C 2017, 5, 8622–8653;
- 26cS. Grigalevicius, Synth. Met. 2006, 156, 1–12.
- 27
- 27aS. H. Park, A. Roy, S. Beaupré, S. Cho, N. Coates, J. S. Moon, D. Moses, M. Leclerc, K. Lee, A. J. Heeger, Nat. Photonics 2009, 3, 297;
- 27bG. Sathiyan, E. K. T. Sivakumar, R. Ganesamoorthy, R. Thangamuthu, P. Sakthivel, Tetrahedron Lett. 2016, 57, 243–252;
- 27cF. Dumur, Org. Electron. 2015, 25, 345–361;
- 27dJ. Li, A. C. Grimsdale, Chem. Soc. Rev. 2010, 39, 2399–2410.
- 28Y.-Y. Zhang, H. Zhou, P. Wu, H.-R. Zhang, J.-J. Xu, H.-Y. Chen, Anal. Chem. 2014, 86, 8657–8664.
- 29J. M. Oliva, N. L. Allan, P. v. R. Schleyer, C. Viñas, F. Teixidor, J. Am. Chem. Soc. 2005, 127, 13538–13547.
- 30K. Hosoi, S. Inagi, T. Kubo, T. Fuchigami, Chem. Commun. 2011, 47, 8632–8634.