Reversible Hydrogen Uptake/Release over a Sodium Phenoxide–Cyclohexanolate Pair
Dedicated to the Dalian Institute of Chemical Physics, Chinese Academy of Sciences on the occasion of its 70th anniversary
Graphical Abstract
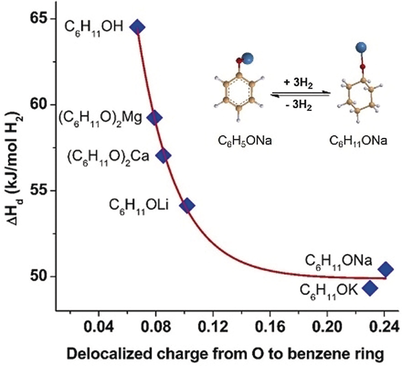
Optimized storage: The metalation of the phenoxide–cyclohexanolate pair is a successful strategy to optimize the thermodynamic properties of this type of hydrogen storage material (see figure). In experiments the sodiated pair desorbed hydrogen at 413 K (373 K) in the solid state (aqueous solution), whereas hydrogenation could be accomplished at temperatures as low as 202 K.
Abstract
Hydrogen uptake and release in arene–cycloalkane pairs provide an attractive opportunity for on-board and off-board hydrogen storage. However, the efficiency of arene–cycloalkane pairs currently is limited by unfavorable thermodynamics for hydrogen release. It is shown here that the thermodynamics can be optimized by replacement of H in the -OH group of cyclohexanol and phenol with alkali or alkaline earth metals. The enthalpy change upon dehydrogenation decreases substantially, which correlates with the delocalization of the oxygen electron to the benzene ring in phenoxides. Theoretical calculations reveal that replacement of H with a metal leads to a reduction of the HOMO–LUMO energy gap and elongation of the C−H bond in the α site in cyclohexanolate, which indicates that the cyclohexanol is activated upon metal substitution. The experimental results demonstrate that sodium phenoxide–cyclohexanolate, an air- and water-stable pair, can desorb hydrogen at ca. 413 K and 373 K in the solid form and in an aqueous solution, respectively. Hydrogenation, on the other hand, is accomplished at temperatures as low as 303 K.