Leveraging γ-Glutamyl Transferase To Direct Cytotoxicity of Copper Dithiocarbamates against Prostate Cancer Cells
Dr. Subha Bakthavatsalam
Duke University, Department of Chemistry, POB 90346, Durham, 27708 USA
Search for more papers by this authorDr. Mark L. Sleeper
Duke University, Department of Chemistry, POB 90346, Durham, 27708 USA
Search for more papers by this authorAzim Dharani
Duke University, Department of Chemistry, POB 90346, Durham, 27708 USA
Search for more papers by this authorDr. Daniel J. George
Division of Medical Oncology Department of, Medicine Duke Cancer Institute, Durham, 27708 USA
Search for more papers by this authorDr. Tian Zhang
Division of Medical Oncology Department of, Medicine Duke Cancer Institute, Durham, 27708 USA
Search for more papers by this authorCorresponding Author
Katherine J. Franz
Duke University, Department of Chemistry, POB 90346, Durham, 27708 USA
Search for more papers by this authorDr. Subha Bakthavatsalam
Duke University, Department of Chemistry, POB 90346, Durham, 27708 USA
Search for more papers by this authorDr. Mark L. Sleeper
Duke University, Department of Chemistry, POB 90346, Durham, 27708 USA
Search for more papers by this authorAzim Dharani
Duke University, Department of Chemistry, POB 90346, Durham, 27708 USA
Search for more papers by this authorDr. Daniel J. George
Division of Medical Oncology Department of, Medicine Duke Cancer Institute, Durham, 27708 USA
Search for more papers by this authorDr. Tian Zhang
Division of Medical Oncology Department of, Medicine Duke Cancer Institute, Durham, 27708 USA
Search for more papers by this authorCorresponding Author
Katherine J. Franz
Duke University, Department of Chemistry, POB 90346, Durham, 27708 USA
Search for more papers by this authorGraphical Abstract
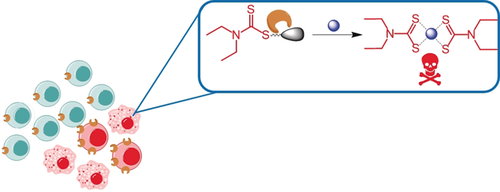
Chelator in disguise: Dithiocarbamate, a metal ion chelator, is masked for its ability to bind to CuII with a γ-glutamyl transpeptidase (GGT) enzyme trigger. Activated by GGT overexpressed in cancer cells, the chelator is unveiled to form cytotoxic Cu complexes in situ selectively in GGT-expressing cancer cells.
Abstract
A prodrug approach is presented to direct copper-dependent cytotoxicity to prostate cancer cells. The prochelator GGTDTC requires activation by γ-glutamyl transferase (GGT) to release the metal chelator diethyldithiocarbamate from a linker that masks its thiol reactivity and metal binding properties. In vitro studies demonstrated successful masking of copper binding as well as clean liberation of the chelator by GGT. GGTDTC was stable to non-specific degradation when incubated with a series of prostate cancer and normal cell lines, with selective release of diethyldithiocarbamate only occurring in cells with measurable GGT activity. The antiproliferative efficacy of the prochelator correlated with cellular GGT activity, with 24 h inhibitory concentrations ranging from 800 nm in prostate cancer lines 22Rv1 and LNCaP to over 15 μm in normal prostate PWR-1E cells. These findings underscore a new strategy to leverage the amplified copper metabolism of prostate cancer by conditional activation of a metal-binding pharmacophore.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201807582-sup-0001-misc_information.pdf1.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. Beltran, D. Prandi, J. M. Mosquera, M. Benelli, L. Puca, J. Cyrta, C. Marotz, E. Giannopoulou, B. V. Chakravarthi, S. Varambally, S. A. Tomlins, D. M. Nanus, S. T. Tagawa, E. M. Van Allen, O. Elemento, A. Sboner, L. A. Garraway, M. A. Rubin, F. Demichelis, Nat. Med. 2016, 22, 298–305;
- 1bR. R. Aggarwal, F. Y. Feng, E. J. Small, Oncology 2017, 31, 467–474.
- 2
- 2aM. A. Cater, H. B. Pearson, K. Wolyniec, P. Klaver, M. Bilandzic, B. M. Paterson, A. I. Bush, P. O. Humbert, S. La Fontaine, P. S. Donnelly, Y. Haupt, ACS Chem. Biol. 2013, 8, 1621–1631;
- 2bD. Denoyer, H. B. Pearson, S. A. S. Clatworthy, Z. M. Smith, P. S. Francis, R. M. Llanos, I. Volitakis, W. A. Phillips, P. M. Meggyesy, S. Masaldan, M. A. Cater, Oncotarget 2016, 7, 37064–37080;
- 2cM. Wehbe, A. W. Y. Leung, M. J. Abrams, C. Orvig, M. B. Bally, Dalton Trans. 2017, 46, 10758–10773.
- 3
- 3aA. Piccardo, F. Paparo, M. Puntoni, S. Righi, G. Bottoni, L. Bacigalupo, S. Zanardi, A. DeCensi, G. Ferrarazzo, M. Gambaro, F. G. Ruggieri, F. Campodonico, L. Tomasello, L. Timossi, S. Sola, E. Lopci, M. Cabria, J. Nucl. Med. 2018, 59, 444–451;
- 3bH. Cai, J.-s. Wu, O. Muzik, J.-T. Hsieh, R. J. Lee, F. Peng, J. Nucl. Med. 2014, 55, 622–628;
- 3cE. Capasso, S. Durzu, S. Piras, S. Zandieh, P. Knoll, A. Haug, M. Hacker, C. Meleddu, S. Mirzaei, Ann. Nucl. Med. 2015, 29, 482–488;
- 3dF. Peng, X. Lu, J. Janisse, O. Muzik, A. F. Shields, J. Nucl. Med. 2006, 47, 1649–1652.
- 4R. Safi, E. R. Nelson, S. K. Chitneni, K. J. Franz, D. J. George, M. R. Zalutsky, D. P. McDonnell, Cancer Res. 2014, 74, 5819–5831.
- 5
- 5aV. Koppaka, D. C. Thompson, Y. Chen, M. Ellermann, K. C. Nicolaou, R. O. Juvonen, D. Petersen, R. A. Deitrich, T. D. Hurley, V. Vasiliou, Pharmacol. Rev. 2012, 64, 520–539;
- 5bB. Johansson, Acta Psychiatr. Scand. Suppl. 1992, 369, 15–26.
- 6
- 6aD. Cen, D. Brayton, B. Shahandeh, F. L. Meyskens, P. J. Farmer, J. Med. Chem. 2004, 47, 6914–6920;
- 6bD. J. Lewis, P. Deshmukh, A. A. Tedstone, F. Tuna, P. O'Brien, Chem. Commun. 2014, 50, 13334–13337;
- 6cP. E. Tawari, Z. Wang, M. Najlah, C. W. Tsang, V. Kannappan, P. Liu, C. McConville, B. He, A. L. Armesilla, W. Wang, Toxicol. Res. 2015, 4, 1439–1442.
- 7
- 7aD. Cen, R. I. Gonzalez, J. A. Buckmeier, R. S. Kahlon, N. B. Tohidian, F. L. Meyskens, Mol. Cancer Therap. 2002, 1, 197;
- 7bC. Conticello, D. Martinetti, L. Adamo, S. Buccheri, R. Giuffrida, N. Parrinello, L. Lombardo, G. Anastasi, G. Amato, M. Cavalli, A. Chiarenza, R. De Maria, R. Giustolisi, M. Gulisano, F. Di Raimondo, Intl. J. Cancer 2012, 131, 2197–2203.
- 8Z. Skrott, M. Mistrik, K. K. Andersen, S. Friis, D. Majera, J. Gursky, T. Ozdian, J. Bartkova, Z. Turi, P. Moudry, M. Kraus, M. Michalova, J. Vaclavkova, P. Dzubak, I. Vrobel, P. Pouckova, J. Sedlacek, A. Miklovicova, A. Kutt, J. Li, J. Mattova, C. Driessen, Q. P. Dou, J. Olsen, M. Hajduch, B. Cvek, R. J. Deshaies, J. Bartek, Nature 2017, 552, 194–199.
- 9
- 9aK. Iljin, K. Ketola, P. Vainio, P. Halonen, P. Kohonen, V. Fey, R. C. Grafström, M. Perälä, O. Kallioniemi, Clin. Cancer Res. 2009, 15, 6070–6078;
- 9bH. L. Wiggins, J. M. Wymant, F. Solfa, S. E. Hiscox, K. M. Taylor, A. D. Westwell, A. T. Jones, Biochem. Pharmacol. 2015, 93, 332–342;
- 9cJ. L. Allensworth, M. K. Evans, F. Bertucci, A. J. Aldrich, R. A. Festa, P. Finetti, N. T. Ueno, R. Safi, D. P. McDonnell, D. J. Thiele, S. Van Laere, G. R. Devi, Mol. Oncol. 2015, 9, 1155–1168;
- 9dB. W. Morrison, N. A. Doudican, K. R. Patel, S. J. Orlow, Melanoma Res. 2010, 20, 11–20.
- 10
- 10aS. Mohapatra, M. R. Sahoo, N. Rath, Gen Hosp Psychiatry 2015, 37, 97.e95–97.e96;
- 10bA. M. G. Farci, S. Piras, M. Murgia, A. Chessa, A. Restivo, G. L. Gessa, R. Agabio, Eat Behav 2015, 16, 84–87;
- 10cK. K. Kumar, S. Bondade, F. A. Sattar, N. Singh, Indian J. Psychol. Med. 2016, 38, 344–347;
- 10dA. T. Tran, R. A. Rison, S. R. Beydoun, J. Med. Case Rep. 2016, 10, 72.
- 11M. T. Schweizer, J. Lin, A. Blackford, A. Bardia, S. King, A. J. Armstrong, M. A. Rudek, S. Yegnasubramanian, M. A. Carducci, Prostate Cancer Prostatic Dis. 2013, 16, 357–361.
- 12
- 12aH. Fasehee, R. Dinarvand, A. Ghavamzadeh, M. Esfandyari-Manesh, H. Moradian, S. Faghihi, S. H. Ghaffari, J. Nanobiotech. 2016, 14, 32;
- 12bZ. Wang, J. Tan, C. McConville, V. Kannappan, P. E. Tawari, J. Brown, J. Ding, A. L. Armesilla, J. M. Irache, Q.-B. Mei, Y. Tan, Y. Liu, W. Jiang, X.-W. Bian, W. Wang, Nanomed: NBM 2017, 13, 641–657.
- 13
- 13aQ. Wang, K. J. Franz, Acc. Chem. Res. 2016, 49, 2468–2477;
- 13bJ. M. Zaengle-Barone, A. C. Jackson, D. M. Besse, B. Becken, M. Arshad, P. C. Seed, K. J. Franz, ACS Infect Dis. 2018, 0, 0;
- 13cP. Hašková, H. Jansová, J. Bureš, M. Macháček, A. Jirkovská, K. J. Franz, P. Kovaříková, T. Šimůnek, Toxicol 2016, 371, 17–28;
- 13dA. M. Pujol, M. Cuillel, A. S. Jullien, C. Lebrun, D. Cassio, E. Mintz, C. Gateau, P. Delangle, Angew. Chem. Int. Ed. 2012, 51, 7445–7448;
- 13eE. A. Akam, R. D. Utterback, J. R. Marcero, H. A. Dailey, E. Tomat, J. Inorg. Biochem. 2018, 180, 186–193.
- 14
- 14aM. H. Hanigan, Adv. Cancer Res. 2014, 122, 103–141;
- 14bA. Corti, M. Franzini, A. Paolichhi, A. Pompella, Anticancer Res. 2010, 30, 1169–1181.
- 15
- 15aJ. B. King, M. B. West, P. F. Cook, M. H. Hanigan, J. Biol. Chem. 2009, 284, 9059–9065;
- 15bM. H. Hanigan, T. Macdonald, (Ed.: V. The University of Virginia (Charlottesville)), United States, 1998;
- 15cE. E. Ramsay, P. J. Dilda, Front Pharmacol. 2014, 5, 181;
- 15dY. Miyata, T. Ishizawa, M. Kamiya, S. Yamashita, K. Hasegawa, A. Ushiku, J. Shibahara, M. Fukayama, Y. Urano, N. Kokudo, Sci. Rep. 2017, 7, 3542;
- 15eZ. Luo, L. Feng, R. An, G. Duan, R. Yan, H. Shi, J. He, Z. Zhou, C. Ji, H. Y. Chen, D. Ye, Chem. Eur. J. 2017, 23, 14778–14785.
- 16
- 16aP. L. Carl, P. K. Chakravarty, J. A. Katzenellenbogen, J. Med. Chem. 1981, 24, 479–480;
- 16bH. Hagen, P. Marzenell, E. Jentzsch, F. Wenz, M. R. Veldwijk, A. Mokhir, J. Med. Chem. 2012, 55, 924–934;
- 16cF. Kielar, M. E. Helsel, Q. Wang, K. J. Franz, Metallomics 2012, 4, 899–909.
- 17S. Wickham, N. Regan, M. B. West, J. Thai, P. F. Cook, S. S. Terzyan, P. K. Li, M. H. Hanigan, Biochem. J. 2013, 450, 547–557.