Syntheses and Structures of Ruthenium Complexes Containing a Ru-H-Tl Three-Center–Two-Electron Bond
Dr. Wei Bai
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorJing-Xuan Zhang
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorDr. Ting Fan
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorDr. Sunny Kai San Tse
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorDr. Wangge Shou
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorDr. Herman H. Y. Sung
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorCorresponding Author
Prof. Dr. Ian D. Williams
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenyang Lin
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorCorresponding Author
Prof. Dr. Guochen Jia
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorDr. Wei Bai
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorJing-Xuan Zhang
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorDr. Ting Fan
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorDr. Sunny Kai San Tse
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorDr. Wangge Shou
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorDr. Herman H. Y. Sung
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorCorresponding Author
Prof. Dr. Ian D. Williams
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhenyang Lin
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorCorresponding Author
Prof. Dr. Guochen Jia
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong
Search for more papers by this authorGraphical Abstract
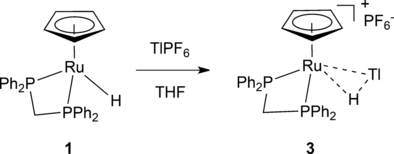
Although η2-H−EXn σ-complexes are well-known when E is a Group 13 or 14 element (E=B, Al, Ga, C, Si, Ge, Sn), η2-H−EXn σ-complexes where E is a period 6 main-group element were previously unknown. Now the synthesis and structural characterization is presented of ruthenium σ-complexes [CpRu(PP)(η2-(H-Tl))]+ (PP=dppm, dppe), which contain TlH, a hydride of a period-6 main group element.
Abstract
Treatment of CpRuH(PP) (PP=dppm, dppe) with TlPF6 produced [CpRu(H)(Tl)(PP)]PF6. X-ray diffraction and computational studies suggest that the complexes contain a Ru−H−Tl 3c–2e bond and can be viewed as the first σ-complexes of period 6 main-group hydrides [CpRu{η2-(H−Tl)}(PP)]PF6 or [Tl{η2-H−RuCp(PP)}]PF6. The complexes can be stored as a solid at room temperature for days without appreciable decomposition, but are unstable in solution and evolved to the trimetallic complexes [{CpRu(PP)}2(μ-Tl)]PF6.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201807174-sup-0001-misc_information.pdf1.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For general reviews, see for example:
- 1aR. H. Crabtree, Angew. Chem. Int. Ed. Engl. 1993, 32, 789–805; Angew. Chem. 1993, 105, 828–845;
- 1bG. J. Kubas, Metal Dihydrogen and σ-Bond Complexes: Structure, Theory and Reactivity, Kluwer Academic/Plenum Press, New York, 2001;
- 1c Recent Advances in Hydride Chemistry (Eds.: ), Elsevier, Amsterdam, 2001.
- 2For recent reviews, see for example:
- 2aR. H. Crabtree, Chem. Rev. 2016, 116, 8750–8769;
- 2bM. A. Esteruelas, A. M. López, M. Oliván, Chem. Rev. 2016, 116, 8770–8847;
- 2cR. H. Morris, Chem. Rev. 2016, 116, 8588–8654;
- 2dG. J. Kubas, Chem. Rev. 2007, 107, 4152–4205.
- 3For recent work, see for example:
- 3aJ. M. Goldberg, K. I. Goldberg, D. M. Heinekey, S. A. Burgess, D. B. Lao, J. C. Linehan, J. Am. Chem. Soc. 2017, 139, 12638–12646;
- 3bL. Szatkowski, M. B. Hall, Inorg. Chem. 2017, 56, 9653–9659;
- 3cR. P. Yu, J. M. Darmon, S. P. Semproni, Z. R. Turner, P. J. Chirik, Organometallics 2017, 36, 4341–4343;
- 3dM. V. Vollmer, J. Xie, C. C. Lu, J. Am. Chem. Soc. 2017, 139, 6570–6573;
- 3eE. Suárez, P. Plou, D. G. Gusev, M. Martín, E. Sola, Inorg. Chem. 2017, 56, 7190–7199;
- 3fM. A. Esteruelas, C. García-Yebra, J. Martín, E. Oñate, Inorg. Chem. 2017, 56, 676–683.
- 4For reviews of Group 13 hydride σ-complexes, see:
- 4aM. J. Butler, M. R. Crimmin, Chem. Commun. 2017, 53, 1348–1365;
- 4bI. M. Riddlestone, J. A. B. Abdalla, S. Aldridge, Adv. Organomet. Chem. 2015, 63, 1–38;
- 4cT. Muraoka, K. Ueno, Coord. Chem. Rev. 2010, 254, 1348–1355.
- 5For recent work, see for example:
- 5aM. A. Esteruelas, I. Fernández, C. García-Yebra, J. Martín, E. Oñate, Organometallics 2017, 36, 2298–2307;
- 5bN. Lalaoui, T. Woods, T. B. Rauchfuss, G. Zampella, Organometallics 2017, 36, 2054–2057;
- 5cA. Ramaraj, K. Hari, K. Reddy, H. Keil, R. Herbst-Irmer, D. Stalke, E. D. Jemmis, B. R. Jagirdar, Organometallics 2017, 36, 2736–2745;
- 5dI. E. Golub, O. A. Filippov, N. V. Belkova, E. I. Gutsul, L. M. Epstein, A. Rossin, M. Peruzzini, E. S. Shubina, Eur. J. Inorg. Chem. 2017, 4673–4682;
- 5eS. Todisco, L. Luconi, G. Giambastiani, A. Rossin, M. Peruzzini, I. E. Golub, O. A. Filippov, N. V. Belkova, E. S. Shubina, Inorg. Chem. 2017, 56, 4296–4307.
- 6For recent work, see for example:
- 6aA. C. Brown, A. B. Altman, T. D. Lohrey, S. Hohloch, J. Arnold, Chem. Sci. 2017, 8, 5153–5160;
- 6bA. Hicken, A. J. P. White, M. R. Crimmin, Inorg. Chem. 2017, 56, 8669–8682;
- 6cJ. Weßing, C. Göbel, B. Weber, C. Gemel, R. A. Fischer, Inorg. Chem. 2017, 56, 3517–3525;
- 6dO. Ekkert, A. J. P. White, H. Toms, M. R. Crimmin, Chem. Sci. 2015, 6, 5617–5622.
- 7For recent work, see for example:
- 7aJ. A. B. Abdalla, A. Caise, C. P. Sindlinger, R. Tirfoin, A. L. Thompson, A. J. Edwards, S. Aldridge, Nat. Chem. 2017, 9, 1256–1262;
- 7bJ. Turner, J. A. B. Abdalla, J. I. Bates, R. Tirfoin, M. J. Kelly, N. Phillips, S. Aldridge, Chem. Sci. 2013, 4, 4245–4250.
- 8For recent reviews, see for example:
- 8aA. S. Weller, F. M. Chadwick, A. I. McKay, Adv. Organomet. Chem. 2016, 66, 223–276;
- 8bR. D. Young, Chem. Eur. J. 2014, 20, 12704–12718.
- 9For recent work, see for example:
- 9aF. M. Chadwick, A. I. McKay, A. J. Martinez-Martinez, N. H. Rees, T. Krämer, S. A. Macgregor, A. S. Weller, Chem. Sci. 2017, 8, 6014–6029;
- 9bA. I. McKay, T. Krämer, N. H. Rees, A. L. Thompson, K. E. Christensen, S. A. Macgregor, A. S. Weller, Organometallics 2017, 36, 22–25;
- 9cH. M. Yau, A. I. McKay, H. Hesse, R. Xu, M. He, C. E. Holt, G. E. Ball, J. Am. Chem. Soc. 2016, 138, 281–288;
- 9dF. M. Chadwick, N. H. Rees, A. S. Weller, T. Krämer, M. Iannuzzi, S. A. Macgregor, Angew. Chem. Int. Ed. 2016, 55, 3677–3681; Angew. Chem. 2016, 128, 3741–3745.
- 10For recent reviews, see for example:
- 10aJ. Y. Corey, Chem. Rev. 2016, 116, 11291–11435;
- 10bJ. Y. Corey, Chem. Rev. 2011, 111, 863–1071;
- 10cS. Lachaize, S. Sabo-Etienne, Eur. J. Inorg. Chem. 2006, 2115–2127;
- 10dG. I. Nikonov, Adv. Organomet. Chem. 2005, 53, 217–309.
- 11For recent work, see for example:
- 11aP. Ríos, J. Díez, J. López-Serrano, A. Rodríguez, S. Conejero, Chem. Eur. J. 2016, 22, 16791–16795;
- 11bJ. Kim, Y. Kim, I. Sinha, K. Park, S. H. Kim, Y. Lee, Chem. Commun. 2016, 52, 9367–9370;
- 11cV. H. Mai, I. Korobkov, G. I. Nikonov, Organometallics 2016, 35, 936–942;
- 11dM. Nagaoka, H. Tsuruda, M. Amako, H. Suzuki, T. Takao, Angew. Chem. Int. Ed. 2015, 54, 14871–14874; Angew. Chem. 2015, 127, 15084–15087.
- 12For recent work, see for example:
- 12aK. A. Smart, E. Mothes-Martin, L. Vendier, R. N. Perutz, M. Grellier, S. Sabo-Etienne, Organometallics 2015, 34, 4158–4163;
- 12bN. Ochi, T. Matsumoto, T. Dei, Y. Nakao, H. Sato, K. Tatsumi, S. Sakaki, Inorg. Chem. 2015, 54, 576–585.
- 13For recent work, see for example:
- 13aA. Koppaka, B. Captain, Inorg. Chem. 2016, 55, 2679–2681;
- 13bA. Koppaka, V. Yempally, L. Zhu, G. C. Fortman, M. Temprado, C. D. Hoff, B. Captain, Inorg. Chem. 2016, 55, 307–321;
- 13cS. M. Rummelt, K. Radkowski, D.-A. Roşca, A. Fürstner, J. Am. Chem. Soc. 2015, 137, 5506–5519;
- 13dC. P. Sindlinger, L. Wesemann, Chem. Commun. 2015, 51, 11421–11424.
- 14See for example:
- 14aA. V. Titov, N. S. Mosyagin, A. B. Alkseyev, Int. J. Quantum Chem. 2001, 81, 409–421;
- 14bY. J. Choi, Y. K. Han, Y. S. Lee, J. Chem. Phys. 2001, 115, 3448–3453;
- 14cK. A. Peterson, J. Chem. Phys. 2003, 119, 11099–11112;
- 14dY. J. Choi, Y. S. Lee, J. Chem. Phys. 2003, 119, 2014–2020;
- 14eF. Wang, T. Ziegler, E. van Lenthe, S. van Gisbergen, E. J. Baerends, J. Chem. Phys. 2005, 122, 204103;
- 14fM. Abe, T. Nakajima, K. Hirao, J. Chem. Phys. 2006, 125, 234110;
- 14gA. Devarajan, A. Gaenko, J. Autschbach, J. Chem. Phys. 2009, 130, 194102;
- 14hI. Kim, Y. C. Park, H. Kim, Y. S. Lee, Chem. Phys. 2012, 395, 115–121.
- 15
- 15aR. D. Urban, A. H. Bahnmaier, U. Magg, H. Jones, Chem. Phys. Lett. 1989, 158, 443–446;
- 15bX. F. Wang, L. Andrews, J. Phys. Chem. A 2004, 108, 3396–3402;
- 15cL. Andrews, X. Wang, Angew. Chem. Int. Ed. 2004, 43, 1706–1709; Angew. Chem. 2004, 116, 1738–1741.
- 16
- 16aD. V. Gutsulyak, L. G. Kuzmina, J. A. K. Howard, S. F. Vyboishchikov, G. I. Nikonov, J. Am. Chem. Soc. 2008, 130, 3732–3733;
- 16bT. Y. Lee, L. Dang, Z. Zhou, C. H. Yeung, Z. Lin, C. P. Lau, Eur. J. Inorg. Chem. 2010, 5675–5684;
- 16cM. E. Fasulo, E. Calimano, J. M. Buchanan, T. D. Tilley, Organometallics 2013, 32, 1016–1028.
- 17M. C. Lipke, T. D. Tilley, Angew. Chem. Int. Ed. 2012, 51, 11115–11121; Angew. Chem. 2012, 124, 11277–11283.
- 18F. M. Conroy-Lewis, S. J. Simpson, J. Chem. Soc. Chem. Commun. 1987, 1675–1676.
- 19CCDC 936703, 936704, and 1850038 (3–5) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 20A. Bondi, J. Phys. Chem. 1964, 68, 441–451.
- 21S. Z. Hu, Z. H. Zhou, Z. H. Robertson, Z. Kristallogr. 2009, 224, 375–376.
- 22P. Barthazy, A. Togni, A. Mezzetti, Organometallics 2001, 20, 3472–3477.
- 23C. Becker, I. Kieltsch, D. Broggini, A. Mezzetti, Inorg. Chem. 2003, 42, 8417–8429.
- 24I. Bytheway, C. S. Griffith, G. A. Koutsantonis, B. W. Skelton, A. H. White, Eur. J. Inorg. Chem. 2007, 3240–3246.
- 25J. C. Jeffery, P. A. Jelliss, Y. H. Liao, F. G. A. Stone, J. Organomet. Chem. 1998, 551, 27–36.
- 26See for example:
- 26aD. D. Ellis, S. M. Couchman, J. C. Jeffery, J. M. Malget, F. G. A. Stone, Inorg. Chem. 1999, 38, 2981–2988;
- 26bA. Albinati, L. M. Venanzi, G. Wang, Inorg. Chem. 1993, 32, 3660–3669.
- 27See for example:
- 27aB. D. Alexander, M. P. Gomez-Sal, P. R. Gannon, C. A. Blaine, P. D. Boyle, A. M. Mueting, L. H. Pignolet, Inorg. Chem. 1988, 27, 3301–3308;
- 27bA. Antiñolo, F. A. Jalon, A. Otero, M. Fajardo, B. Chaudret, F. Lahoz, J. A. Lopez, J. Chem. Soc. Dalton Trans. 1991, 1861–1866.
- 28M. J. Taylor, P. J. Brothers, Chemistry of Aluminium, Gallium and Thallium (Ed.: ), Chapman & Hall, London, 1993, pp. 111–247.
- 29U. Schubert, E. Kunz, B. Harkers, J. Willnecker, J. Meyer, J. Am. Chem. Soc. 1989, 111, 2572–2574.
- 30J. L. Vincent, S. Luo, B. L. Scott, R. Butcher, C. J. Unkefer, C. J. Burns, G. J. Kubasm, A. Lledos, F. Maseras, J. Tomas, Organometallics 2003, 22, 5307–5323.
- 31W. T. Klooster, T. F. Koetzle, G. Jia, T. P. Fong, R. H. Morris, A. Albinati, J. Am. Chem. Soc. 1994, 116, 7677–7681.
- 32E. J. Fernández, J. M. Lopez-de-Luzuriaga, M. Monge, M. Montiel, M. E. Olmos, J. Perez, Inorg. Chem. 2004, 43, 3573–3581.
- 33J. F. Hinton, Bull. Magn. Reson. 1992, 13, 90–108.
- 34See for example:
- 34aV. J. Catalano, B. B. Bennett, R. L. Yson, B. C. Noll, J. Am. Chem. Soc. 2000, 122, 10056–10062;
- 34bN. Oberbeckmann-Winter, P. Braunstein, R. Welter, Organometallics 2004, 23, 6311–6318;
- 34cJ. Forniés, A. Garcia, E. Lalinde, M. T. Moreno, Inorg. Chem. 2008, 47, 3651–3660;
- 34dS. Jamali, M. M. Ashtiani, Z. Jamshidi, E. Lalinde, M. T. Moreno, H. Samouei, E. Escudero-Adán, J. Benet-Buchholz, Inorg. Chem. 2013, 52, 10729–10731;
- 34eH. Braunschweig, R. D. Dewhurst, F. Hupp, C. Schneider, Chem. Commun. 2014, 50, 15685–15688.
- 35Examples of complexes with observable 2J(TlP) coupling are still rare; see for example:
- 35aV. J. Catalano, B. B. Bennett, H. M. Kar, B. C. Noll, J. Am. Chem. Soc. 1999, 121, 10235–10236;
- 35bS. Jamali, R. Ghazfar, E. Lalinde, Z. Jamshidi, H. Samouei, H. R. Shahsavari, M. T. Moreno, E. Escudero-Adan, J. Benet-Buchholz, D. Milic, Dalton Trans. 2014, 43, 1105–1116;
- 35cV. J. Catalano, M. A. Malwitz, J. Am. Chem. Soc. 2004, 126, 6560–6561.
- 36Because of the large line widths and nearly identical gyromagnetic ratios, the individual couplings to 203Tl and 205Tl could not be differentiated.
- 37V. P. Tatasov, S. I. Bakum, J. Magn. Reson. 1975, 18, 64–68.
- 38The assignments of the ν(Ru–H) bands were confirmed by the absence of the IR bands in the IR spectra of [CpRu{η2-(D-Tl)}(PP)]PF6. DFT calculations predict that ν(Ru–D) and ν(Tl–D) of [CpRu{η2-(D−Tl)}(dppm)]PF6 should appear at 1348 and 562 cm−1, respectively, and that ν(Ru–D) and ν(Tl–D) of [CpRu{η2-(D−Tl)}(dppe)]PF6 should appear at 1334 and 601 cm−1, respectively. Unfortunately, these bands could not be confidently identified, because these regions are filled with the IR bands arising from other ligands.
- 39For example:
- 39aA. J. Downs, C. R. Pulham, Chem. Soc. Rev. 1994, 23, 175–184;
- 39bM. G. Gardiner, C. L. Raston, Coord. Chem. Rev. 1997, 166, 1–34;
- 39cA. J. Downs, Coord. Chem. Rev. 1999, 189, 59–100;
- 39dJ. A. Jegier, W. L. Gladfelter, Coord. Chem. Rev. 2000, 206–207, 631–650;
- 39eS. Aldridge, A. J. Downs, Chem. Rev. 2001, 101, 3305–3366.
- 40Examples of agostic thallium hydrogen bond <2.5 Å:
- 40aC. Dowling, P. Ghosh, G. Parkin, Polyhedron 1997, 16, 3469–3473;
- 40bT. J. Boyle, C. A. Zechmann, T. M. Alam, M. A. Rodriguez, C. A. Hijar, B. L. Scott, Inorg. Chem. 2002, 41, 946–957;
- 40cW. Z. Chen, F. H. Liu, K. Matsumoto, J. Autschbach, B. Le Guennic, T. Ziegler, M. Maliarik, J. Glaser, Inorg. Chem. 2006, 45, 4526–4536.
- 41C. Bianchini, D. Masi, K. Linn, C. Mealli, M. Peruzzini, F. Zanobini, Inorg. Chem. 1992, 31, 4036–4037.