Gold-Catalyzed Dimerization of Diarylalkynes: Direct Access to Azulenes
M. Sc. Vanessa Claus
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorMichael Schukin
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorSiegfried Harrer
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorDr. Matthias Rudolph
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorDr. Frank Rominger
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Crystallographic analysis.
Search for more papers by this authorProf. Dr. Abdullah M. Asiri
Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, 21589 Saudi Arabia
Search for more papers by this authorDr. Jin Xie
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. A. Stephen K. Hashmi
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, 21589 Saudi Arabia
Search for more papers by this authorM. Sc. Vanessa Claus
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorMichael Schukin
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorSiegfried Harrer
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorDr. Matthias Rudolph
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorDr. Frank Rominger
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Crystallographic analysis.
Search for more papers by this authorProf. Dr. Abdullah M. Asiri
Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, 21589 Saudi Arabia
Search for more papers by this authorDr. Jin Xie
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. A. Stephen K. Hashmi
Organisch-Chemisches Institut, Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, 21589 Saudi Arabia
Search for more papers by this authorGraphical Abstract
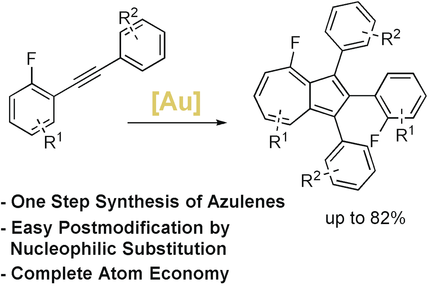
A fully atom-economic gold-catalyzed reaction enables the direct synthesis of substituted azulenes from easily accessible starting materials. A fluorine substituent as a strong +M donor initiates this reaction. The obtained scaffolds can be easily functionalized at the seven-membered ring in a selective manner.
Abstract
We have developed a simple gold-catalyzed procedure for the synthesis of substituted and modifiable azulenes. The azulenes are formed either by the dimerization of push–pull diarylalkynes bearing a fluorine atom in ortho or para position or by the dimerization of a symmetric electron-rich diarylalkyne. In the presence of a cationic gold catalyst, the two alkynes can form a highly reactive vinyl cation. Trapping of this high-energy intermediate by an appropriate aryl unit then delivers substituted azulenes in a single step and in a perfect atom economy.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201805918-sup-0001-misc_information.pdf2.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. Pflästerer, A. S. K. Hashmi, Chem. Soc. Rev. 2016, 45, 1331–1367;
- 1bC. M. Friend, A. S. K. Hashmi, Acc. Chem. Res. 2014, 47, 729–730;
- 1cN. Bongers, N. Krause, Angew. Chem. Int. Ed. 2008, 47, 2178–2181; Angew. Chem. 2008, 120, 2208–2211;
- 1dA. S. K. Hashmi, Chem. Rev. 2007, 107, 3180–3211;
- 1eA. S. K. Hashmi, G. J. Hutchings, Angew. Chem. Int. Ed. 2006, 45, 7896–7936; Angew. Chem. 2006, 118, 8064–8105;
- 1fS. Md. A. Sohel, R.-S. Liu, Chem. Soc. Rev. 2009, 38, 2269–2281.
- 2
- 2aA. M. Asiri, A. S. K. Hashmi, Chem. Soc. Rev. 2016, 45, 4471–4503;
- 2bR. Dorel, A. M. Echavarren, Chem. Rev. 2015, 115, 9028–9072;
- 2cH. Ohno, Isr. J. Chem. 2013, 53, 869–882.
- 3A. S. K. Hashmi, Acc. Chem. Res. 2014, 47, 864–876.
- 4
- 4aA. S. K. Hashmi, I. Braun, P. Nösel, J. Schädlich, M. Wieteck, M. Rudolph, F. Rominger, Angew. Chem. Int. Ed. 2012, 51, 4456–4460; Angew. Chem. 2012, 124, 4532–4536;
- 4bA. S. K. Hashmi, I. Braun, M. Rudolph, F. Rominger, Organometallics 2012, 31, 644–661;
- 4cA. S. K. Hashmi, M. Wieteck, I. Braun, P. Nösel, L. Jongbloed, M. Rudolph, F. Rominger, Adv. Synth. Catal. 2012, 354, 555–562;
- 4dA. S. K. Hashmi, M. Wieteck, I. Braun, M. Rudolph, F. Rominger, Angew. Chem. Int. Ed. 2012, 51, 10633–10637; Angew. Chem. 2012, 124, 10785–10789;
- 4eK. Graf, P. D. Hindenberg, Y. Tokimizu, S. Naoe, M. Rudolph, F. Rominger, H. Ohno, A. S. K. Hashmi, ChemCatChem 2014, 6, 199–204;
- 4fM. H. Vilhelmsen, A. S. K. Hashmi, Chem. Eur. J. 2014, 20, 1901–1908;
- 4gJ. Bucher, T. Stößer, M. Rudolph, F. Rominger, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2015, 54, 1666–1670; Angew. Chem. 2015, 127, 1686–1690;
- 4hC. Yu, B. Chen, T. Zhou, Q. Tian, G. Zhang, Angew. Chem. Int. Ed. 2015, 54, 10903–10907; Angew. Chem. 2015, 127, 11053–11057;
- 4iD. D. Vachhani, M. Galli, J. Jacobs, L. Van Meervelt, E. V. Van der Eycken, Chem. Commun. 2013, 49, 7171–7173.
- 5
- 5aM. M. Hansmann, S. Tšupova, M. Rudolph, F. Rominger, A. S. K. Hashmi, Chem. Eur. J. 2014, 20, 2215–2223;
- 5bY. Wang, A. Yepremyan, S. Ghorai, R. Todd, D. H. Aue, L. Zhang, Angew. Chem. Int. Ed. 2013, 52, 7795–7799; Angew. Chem. 2013, 125, 7949–7953;
- 5cM. M. Hansmann, M. Rudolph, F. Rominger, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2013, 52, 2593–2598; Angew. Chem. 2013, 125, 2653–2659.
- 6T. Wurm, J. Bucher, S. B. Duckworth, M. Rudolph, F. Rominger, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2017, 56, 3364–3368; Angew. Chem. 2017, 129, 3413–3417.
- 7T. Wurm, J. Bucher, M. Rudolph, F. Rominger, A. S. K. Hashmi, Adv. Synth. Catal. 2017, 359, 1637–1642.
- 8
- 8aS. Sun, J. Kroll, Y. Luo, L. Zhang, Synlett 2012, 23, 54–56;
- 8bA. S. K. Hashmi, T. Lauterbach, P. Nösel, M. H. Vilhelmsen, M. Rudolph, F. Rominger, Chem. Eur. J. 2013, 19, 1058–1065;
- 8cN. Endo, M. Kanaura, M. P. Schramm, T. Iwasawa, Eur. J. Org. Chem. 2016, 2514–2521;
- 8dS. Mader, L. Molinari, M. Rudolph, F. Rominger, A. S. K. Hashmi, Chem. Eur. J. 2015, 21, 3910–3913.
- 9V. Weingand, T. Wurm, V. Vethacke, M. C. Dietl, D. Ehjeij, M. Rudolph, F. Rominger, J. Xie, A. S. K. Hashmi, Chem. Eur. J. 2018, 24, 3725–3728.
- 10A. G. Anderson, B. M. Steckler, J. Am. Chem. Soc. 1959, 81, 4941–4946.
- 11J. Michl, E. W. Thulstrup, Tetrahedron 1976, 32, 205–209.
- 12S. V. Shevyakov, H. Li, R. Muthyala, A. E. Asato, J. C. Croney, D. M. Jameson, R. S. H. Liu, J. Phys. Chem. A 2003, 107, 3295–3299.
- 13
- 13aH. Xin, X. Gao, ChemPlusChem 2017, 82, 945–956;
- 13bE. Amir, M. Murai, R. J. Amir, J. S. Cowart, M. L. Chabinyc, C. J. Hawker, Chem. Sci. 2014, 5, 4483–4489;
- 13cT. Wächter, K. J. Scheetz, A. D. Spaeth, M. V. Barybin, M. Zharnikov, J. Phys. Chem. C 2017, 121, 13777–13785;
- 13dA. M. El-Nahas, A. Staykov, K. Yoshizawa, J. Phys. Chem. C 2017, 121, 2504–2511;
- 13eA. M. El-Nahas, A. Staykov, K. Yoshizawa, J. Phys. Chem. C 2016, 120, 9043–9052;
- 13fS. Schmitt, M. Baumgarten, J. Simon, K. Hafner, Angew. Chem. Int. Ed. 1998, 37, 1077–1081;
10.1002/(SICI)1521-3773(19980504)37:8<1077::AID-ANIE1077>3.0.CO;2-R PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 1129–1133;
- 13gY. Yamaguchi, M. Takubo, K. Ogawa, K. Nakayama, T. Koganezawa, H. Katagiri, J. Am. Chem. Soc. 2016, 138, 11335–11343;
- 13hT. Koide, M. Takesue, T. Murafuji, K. Satomi, Y. Suzuki, J. Kawamata, K. Terai, M. Suzuki, H. Yamada, Y. Shiota, K. Yoshizawa, F. Tani, ChemPlusChem 2017, 82, 1010–1014;
- 13iH. Xin, C. Ge, X. Yang, H. Gao, X. Yang, X. Gao, Chem. Sci. 2016, 7, 6701–6705;
- 13jJ. Yao, Z. Cai, Z. Liu, C. Yu, H. Luo, Y. Yang, S. Yang, G. Zhang, D. Zhang, Macromolecules 2015, 48, 2039–2047;
- 13kJ. Xia, B. Capozzi, S. Wei, M. Strange, A. Batra, J. R. Moreno, R. J. Amir, E. Amir, G. C. Solomon, L. Venkataraman, L. M. Campos, Nano Lett. 2014, 14, 2941–2945;
- 13lY. Yamaguchi, K. Ogawa, K.-i. Nakayama, Y. Ohba, H. Katagiri, J. Am. Chem. Soc. 2013, 135, 19095–19098;
- 13mY. Yamaguchi, Y. Maruya, H. Katagiri, K.-i. Nakayama, Y. Ohba, Org. Lett. 2012, 14, 2316–2319;
- 13nT. Shoji, S. Ito, Chem. Eur. J. 2017, 23, 16696–16709.
- 14
- 14aP. M. Gosavi, Y. S. Moroz, I. V. Korendovych, Chem. Commun. 2015, 51, 5347–5350;
- 14bY. S. Moroz, W. Binder, P. Nygren, G. A. Caputo, I. V. Korendovych, Chem. Commun. 2013, 49, 490–492;
- 14cW. Pham, Y. Choi, R. Weissleder, C.-H. Tung, Bioconjugate Chem. 2004, 15, 1403–1407;
- 14dW. Pham, R. Weissleder, C.-H. Tung, Angew. Chem. Int. Ed. 2002, 41, 3659–3662;
10.1002/1521-3773(20021004)41:19<3659::AID-ANIE3659>3.0.CO;2-Q CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 3811–3814;
- 14eG. Loidl, H.-J. Musiol, N. Budisa, R. Huber, S. Poirot, D. Fourmy, L. Moroder, J. Pept. Sci. 2000, 6, 139–144;
10.1002/(SICI)1099-1387(200003)6:3<139::AID-PSC240>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 14fY. Zhou, Y. Zhuang, X. Li, H. Agren, L. Yu, J. Ding, L. Zhu, Chemistry 2017, 23, 7642–7647.
- 15
- 15aM. Yokota, S. Uchibori, H. Hayashi, R. Koyama, K. Kosakai, S. Wakabayashi, T. Tomiyama, Bioorg. Med. Chem. 1996, 4, 575–591;
- 15bH. Nakamura, M. Sekido, Y. Yamamoto, J. Med. Chem. 1997, 40, 2825–2830;
- 15cD. Chen, S. Yu, L. van Ofwegen, P. Proksch, W. Lin, J. Agric. Food Chem. 2012, 60, 112–123;
- 15dJ. Peet, A. Selyutina, A. Bredihhin, Bioorg. Med. Chem. 2016, 24, 1653–1657;
- 15eK. Ikegai, M. Imamura, T. Suzuki, K. Nakanishi, T. Murakami, E. Kurosaki, A. Noda, Y. Kobayashi, M. Yokota, T. Koide, K. Kosakai, Y. Ohkura, M. Takeuchi, H. Tomiyama, M. Ohta, Bioorg. Med. Chem. 2013, 21, 3934–3948.
- 16
- 16aT. Shoji, T. Araki, S. Sugiyama, A. Ohta, R. Sekiguchi, S. Ito, T. Okujima, K. Toyota, J. Org. Chem. 2017, 82, 1657–1665;
- 16bE. H. Ghazvini Zadeh, A. W. Woodward, D. Richardson, M. V. Bondar, K. D. Belfield, Eur. J. Org. Chem. 2015, 2271–2276;
- 16cE. H. Ghazvini Zadeh, S. Tang, A. W. Woodward, T. Liu, M. V. Bondar, K. D. Belfield, J. Mater. Chem. C 2015, 3, 8495–8503;
- 16dE. Amir, R. J. Amir, L. M. Campos, C. J. Hawker, J. Am. Chem. Soc. 2011, 133, 10046–10049;
- 16eM. Porsch, G. Sigl-Seifert, J. Daub, Adv. Mater. 1997, 9, 635–639.
- 17
- 17aK. Hafner, Justus Liebigs Ann. Chem. 1957, 606, 79–89;
- 17bE. H. K. Ziegler, K. Hafner, 1956, US2766304 (A).
- 18T. Nozoe, S. Seto, S. Matsumura, Y. Murase, Bull. Chem. Soc. Jpn. 1962, 35, 1179–1188.
- 19
- 19aC. Lambert, G. Noll, M. Zabel, F. Hampel, E. Schmalzlin, C. Brauchle, K. Meerholz, Chem. Eur. J. 2003, 9, 4232–4239;
- 19bE. Müller, G. Zountsas, Chem.-Ztg. 1974, 98, 41–42;
- 19cE. Müller, G. Zountsas, Chem.-Ztg. 1973, 97, 447.
- 20
- 20aS. Kramer, Y. Odabachian, J. Overgaard, M. Rottländer, F. Gagosz, T. Skrydstrup, Angew. Chem. Int. Ed. 2011, 50, 5090–5094; Angew. Chem. 2011, 123, 5196–5200;
- 20bduring the refereeing of this manuscript, another interesting synthesis of polyannulated azulene-containing structures was published: A. Konishi, A. Morinaga, M. Yasuda, Chem. Eur. J. 2018, 24, 8548–8552.
- 21C.-F. Xu, M. Xu, Y.-X. Jia, C.-Y. Li, Org. Lett. 2011, 13, 1556–1559.
- 22A. Collado, A. Gomez-Suarez, A. R. Martin, A. M. Z. Slawin, S. P. Nolan, Chem. Commun. 2013, 49, 5541–5543.
- 23CCDC 1843375 (2 a′) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 24
- 24aK. Hafner, H. Patzelt, H. Kaiser, Justus Liebigs Ann. Chem. 1962, 656, 24–33;
- 24bM. Makosza, R. Podraza, Eur. J. Org. Chem. 2000, 193–198;
- 24cT. Shoji, Y. Fujiwara, A. Maruyama, M. Maruyama, S. Ito, M. Yasunami, R. Yokoyama, N. Morita, Heterocycles 2015, 90, 85.