Highlight
Polyketide Synthase Modules Redefined
Prof. Adrian T. Keatinge-Clay,
Corresponding Author
Prof. Adrian T. Keatinge-Clay
Department of Molecular Biosciences, The University of Texas at Austin, 100 E. 24th St., Austin, TX, 78712 USA
Search for more papers by this authorProf. Adrian T. Keatinge-Clay,
Corresponding Author
Prof. Adrian T. Keatinge-Clay
Department of Molecular Biosciences, The University of Texas at Austin, 100 E. 24th St., Austin, TX, 78712 USA
Search for more papers by this authorGraphical Abstract
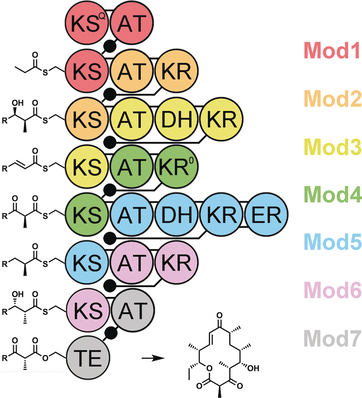
Modular redefinition: A long-standing paradigm in modular polyketide synthase enzymology, namely the definition of a module, has been challenged by Abe and co-workers in their recent study. With this new understanding has emerged renewed hope for engineering these assembly lines to produce new materials and medicines.
References
- 1
- 1aJ. Cortes, S. F. Haydock, G. A. Roberts, D. J. Bevitt, P. F. Leadlay, Nature 1990, 348, 176–178;
- 1bS. Donadio, M. J. Staver, J. B. McAlpine, S. J. Swanson, L. Katz, Science 1991, 252, 675–679;
- 1cA. T. Keatinge-Clay, Nat. Prod. Rep. 2012, 29, 1050–1073;
- 1dJ. Piel, Nat. Prod. Rep. 2010, 27, 996–1047;
- 1eE. J. Helfrich, J. Piel, Nat. Prod. Rep. 2016, 33, 231–316.
- 2L. Zhang, T. Hashimoto, B. Qin, J. Hashimoto, I. Kozone, T. Kawahara, M. Okada, T. Awakawa, T. Ito, Y. Asakawa, M. Ueki, S. Takahashi, H. Osada, T. Wakimoto, H. Ikeda, K. Shin-ya, I. Abe, Angew. Chem. Int. Ed. 2017, 56, 1740–1745; Angew. Chem. 2017, 129, 1766–1771.
- 3T. Maier, M. Leibundgut, N. Ban, Science 2008, 321, 1315–1322.
- 4T. Nguyen, K. Ishida, H. Jenke-Kodama, E. Dittmann, C. Gurgui, T. Hochmuth, S. Taudien, M. Platzer, C. Hertweck, J. Piel, Nat. Biotechnol. 2008, 26, 225–233.
- 5
- 5aK. M. Fisch, C. Gurgui, N. Heycke, S. A. van der Sar, S. A. Anderson, V. L. Webb, S. Taudien, M. Platzer, B. K. Rubio, S. J. Robinson, P. Crews, J. Piel, Nat. Chem. Biol. 2009, 5, 494–501;
- 5bR. Ueoka, A. R. Uria, S. Reiter, T. Mori, P. Karbaum, E. E. Peters, E. J. N. Helfrich, B. I. Morinaka, M. Gugger, H. Takeyama, S. Matsunaga, J. Piel, Nat. Chem. Biol. 2015, 11, 705–714.
- 6M. Jenner, S. Frank, A. Kampa, C. Kohlhass, P. Pöplau, G. S. Briggs, J. Piel, N. J. Oldham, Angew. Chem. Int. Ed. 2013, 52, 1143–1147; Angew. Chem. 2013, 125, 1181–1185.
- 7
- 7aJ. Wu, K. Kinoshita, C. Khosla, D. E. Cane, Biochemistry 2004, 43, 16301–16310;
- 7bX. Guo, T. Liu, Z. Deng, D. E. Cane, Biochemistry 2012, 51, 879–887;
- 7cB. Busch, N. Ueberschaar, S. Behnken, Y. Sugimoto, M. Werneburg, N. Traitcheva, J. He, C. Hertweck, Angew. Chem. Int. Ed. 2013, 52, 5285–5289; Angew. Chem. 2013, 125, 5393–5397.
- 8
- 8aA. Ranganathan, M. Timoney, M. Bycroft, J. Cortés, I. P. Thomas, B. Wilkinson, L. Kellenberger, U. Hanefeld, I. S. Galloway, J. Staunton, P. F. Leadlay, Chem. Biol. 1999, 6, 731–741;
- 8bS. S. Chandran, H. G. Menzella, J. R. Carney, D. V. Santi, Chem. Biol. 2006, 13, 469–474.
- 9A. C. Murphy, H. Hong, S. Vance, R. W. Broadhurst, P. F. Leadlay, Chem. Commun. 2016, 52, 8373–8376.
- 10M. Medema et al., Nat. Chem. Biol. 2015, 11, 625–631.
- 11N. Gaitatzis, B. Silakowski, B. Kunze, G. Nordsiek, H. Blocker, G. Hofle, R. Muller, J. Biol. Chem. 2002, 277, 13082–13090.
- 12
- 12aR. W. Broadhurst, D. Nietlispach, M. P. Wheatcroft, P. F. Leadlay, K. J. Weissman, Chem. Biol. 2003, 10, 723–731;
- 12bJ. R. Whicher, S. S. Smaga, D. A. Hansen, W. C. Brown, W. H. Gerwick, D. H. Sherman, J. L. Smith, Chem. Biol. 2013, 20, 1340–1351.
- 13M. Winn, J. K. Fyans, Y. Zhuo, J. Micklefield, Nat. Prod. Rep. 2016, 33, 317–347.
- 14
- 14aS. Dutta, J. R. Whicher, D. A. Hansen, W. A. Hale, J. A. Chemler, G. R. Congdon, A. R. Narayan, K. Håkansson, D. H. Sherman, J. L. Smith, G. Skiniotis, Nature 2014, 510, 512–517;
- 14bJ. R. Whicher, S. Dutta, D. A. Hansen, W. A. Hale, J. A. Chemler, A. M. Dosey, A. R. Narayan, K. Håkansson, D. H. Sherman, J. L. Smith, G. Skiniotis, Nature 2014, 510, 560–564.
- 15Y. Sugimoto, K. Ishida, N. Traitcheva, B. Busch, H. M. Dahse, C. Hertweck, Chem. Biol. 2015, 22, 229–240.