Amine-Rich Nitrogen-Doped Carbon Nanodots as a Platform for Self-Enhancing Electrochemiluminescence
Serena Carrara
ISIS & icFRC, Université de Strasbourg & CNRS, 8 rue Gaspard Monge, 67000 Strasbourg, France
These authors contributed equally to this work.
Search for more papers by this authorFrancesca Arcudi
ISIS & icFRC, Université de Strasbourg & CNRS, 8 rue Gaspard Monge, 67000 Strasbourg, France
Department of Chemical and Pharmaceutical Sciences, INSTM UdR Trieste, University of Trieste, Via Licio Giorgieri 1, 34127 Trieste, Italy
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Maurizio Prato
Department of Chemical and Pharmaceutical Sciences, INSTM UdR Trieste, University of Trieste, Via Licio Giorgieri 1, 34127 Trieste, Italy
Carbon Nanobiotechnology Laboratory, CIC biomaGUNE, Paseo de Miramón 182, 20009 Donostia-San Sebastian, Spain
Ikerbasque, Basque Foundation for Science, 48013 Bilbao, Spain
Search for more papers by this authorCorresponding Author
Prof. Luisa De Cola
ISIS & icFRC, Université de Strasbourg & CNRS, 8 rue Gaspard Monge, 67000 Strasbourg, France
Search for more papers by this authorSerena Carrara
ISIS & icFRC, Université de Strasbourg & CNRS, 8 rue Gaspard Monge, 67000 Strasbourg, France
These authors contributed equally to this work.
Search for more papers by this authorFrancesca Arcudi
ISIS & icFRC, Université de Strasbourg & CNRS, 8 rue Gaspard Monge, 67000 Strasbourg, France
Department of Chemical and Pharmaceutical Sciences, INSTM UdR Trieste, University of Trieste, Via Licio Giorgieri 1, 34127 Trieste, Italy
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Maurizio Prato
Department of Chemical and Pharmaceutical Sciences, INSTM UdR Trieste, University of Trieste, Via Licio Giorgieri 1, 34127 Trieste, Italy
Carbon Nanobiotechnology Laboratory, CIC biomaGUNE, Paseo de Miramón 182, 20009 Donostia-San Sebastian, Spain
Ikerbasque, Basque Foundation for Science, 48013 Bilbao, Spain
Search for more papers by this authorCorresponding Author
Prof. Luisa De Cola
ISIS & icFRC, Université de Strasbourg & CNRS, 8 rue Gaspard Monge, 67000 Strasbourg, France
Search for more papers by this authorGraphical Abstract
Abstract
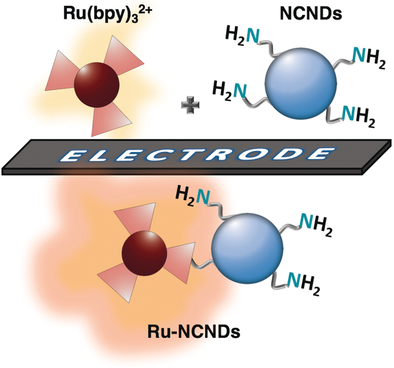
Amine-rich nitrogen-doped carbon nanodots (NCNDs) have been successfully used as co-reactant in electrochemiluminescence (ECL) processes. Primary or tertiary amino groups on NCNDs have been studied as co-reactant sites for Ru(bpy)32+ ECL, showing their eligibility as powerful alternatives to tripropylamine (TPrA). We also report the synthesis and ECL behavior of a new covalently linked hybrid of NCNDs and Ru(bpy)32+. Notably, the NCNDs in the hybrid act both as carrier for ECL labels and as co-reactant for ECL generation. As a result, the hybrid shows a higher ECL emission as compared to the combination of the individual components, suggesting the self-enhancing ECL of the ruthenium complex due to an intramolecular electron transfer process.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201611879-sup-0001-misc_information.pdf2.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, et al., J. Am. Chem. Soc. 2006, 128, 7756–7757.
- 2V. Georgakilas, J. A. Perman, J. Tucek, R. Zboril, Chem. Rev. 2015, 115, 4744–4822.
- 3S. N. Baker, G. A. Baker, Angew. Chem. Int. Ed. 2010, 49, 6726–6744; Angew. Chem. 2010, 122, 6876–6896.
- 4S. Y. Lim, W. Shen, Z. Gao, Chem. Soc. Rev. 2015, 44, 362–381.
- 5P. Roy, P.-C. Chen, A. P. Periasamy, Y.-N. Chen, H.-T. Chang, Mater. Today 2015, 18, 447–458.
- 6
- 6aS. T. Yang, L. Cao, P. G. Luo, F. Lu, X. Wang, H. Wang, M. J. Meziani, Y. Liu, G. Qi, Y. P. Sun, J. Am. Chem. Soc. 2009, 131, 11308–11309;
- 6bF. Arcudi, L. Đorđević, M. Prato, Angew. Chem. Int. Ed. 2017, DOI: 10.1002/anie.201612160; Angew. Chem. 2017, DOI: 10.1002/ange.201612160.
- 7A. J. Bard, Electrogenerated Chemiluminescence, Marcel Dekker, New York, 2004.
10.1201/9780203027011 Google Scholar
- 8M. M. Richter, Chem. Rev. 2004, 104, 3003–3036.
- 9I. Rubinstein, A. J. Bard, J. Am. Chem. Soc. 1981, 103, 512–516.
- 10D. Ege, W. G. Becker, A. J. Bard, Anal. Chem. 1984, 56, 2413–2417.
- 11G. Valenti, E. Rampazzo, S. Bonacchi, L. Petrizza, M. Marcaccio, M. Montalti, L. Prodi, F. Paolucci, J. Am. Chem. Soc. 2016, 138, 15935–15942.
- 12W. Miao, Chem. Rev. 2008, 108, 2506–2553.
- 13Z. Liu, W. Qi, G. Xu, Chem. Soc. Rev. 2015, 44, 3117–3142.
- 14X. Liu, L. Shi, W. Niu, H. Li, G. Xu, Angew. Chem. Int. Ed. 2007, 46, 421–424; Angew. Chem. 2007, 119, 425–428.
- 15P. Liang, L. Dong, M. T. Martin, J. Am. Chem. Soc. 1996, 118, 9198–9199.
- 16P. Li, Z. Jin, M. Zhao, Y. Xu, Y. Guo, D. Xiao, Dalton Trans. 2015, 44, 2208–2216.
- 17H. Wang, Y. He, Y. Chai, R. Yuan, Nanoscale 2014, 6, 10316–10322.
- 18H. Wang, Y. Yuan, Y. Zhuo, Y. Chai, R. Yuan, Anal. Chem. 2016, 88, 2258–2265.
- 19W. Liang, Y. Zhuo, C. Xiong, Y. Zheng, Y. Chai, R. Yuan, Anal. Chem. 2015, 87, 12363–12371.
- 20K. N. Swanick, S. Ladouceur, E. Zysman-Colman, Z. Ding, Angew. Chem. Int. Ed. 2012, 51, 11079–11082; Angew. Chem. 2012, 124, 11241–11244.
- 21Y.-M. Long, L. Bao, J.-Y. Zhao, Z.-L. Zhang, D.-W. Pang, Anal. Chem. 2014, 86, 7224–7228.
- 22L. Li, B. Yu, X. Zhang, T. You, Anal. Chim. Acta 2015, 895, 104–111.
- 23Z. Xu, J. Yu, G. Liu, Sens. Actuators B 2013, 181, 209–214.
- 24F. Arcudi, L. Đorđević, M. Prato, Angew. Chem. Int. Ed. 2016, 55, 2107–2112; Angew. Chem. 2016, 128, 2147–2152.
- 25A. W. Knight, G. M. Greenway, Analyst 1996, 121, 101R.
- 26L. Hu, G. Xu, Chem. Soc. Rev. 2010, 39, 3275.
- 27W. Miao, J.-P. Choi, A. J. Bard, J. Am. Chem. Soc. 2002, 124, 14478–14485.
- 28N. E. Tokel-Takvoryan, R. E. Hemingway, A. J. Bard, J. Am. Chem. Soc. 1973, 95, 6582–6589.
- 29D. M. Hercules, F. E. Lytle, J. Am. Chem. Soc. 1966, 88, 4745–4746.
- 30F. E. Lytle, D. M. Hercules, Photochem. Photobiol. 1971, 13, 123–133.
- 31W. L. Wallace, A. J. Bard, J. Phys. Chem. 1979, 83, 1350–1357.
- 32C. D. Jonah, M. S. Matheson, D. Meisel, J. Am. Chem. Soc. 1978, 100, 1449–1456.
- 33E. Kerr, E. H. Doeven, D. J. D. Wilson, C. F. Hogan, P. S. Francis, Analyst 2016, 141, 62–69.
- 34T. M. Downey, T. A. Nieman, Anal. Chem. 1992, 64, 261–268.
- 35J. K. Leland, M. J. Powell, J. Electrochem. Soc. 1990, 137, 3127.
- 36F. Kanoufi, Y. Zu, A. J. Bard, J. Phys. Chem. B 2001, 105, 210–216.
- 37W. Miao, J.-P. Choi, A. J. Bard, J. Am. Chem. Soc. 2002, 124, 14478–14485.
- 38M. Ameloot, A. U. Acuña, B. Valeur, U. Hasselt, C. Building, Pure Appl. Chem. 2013, 85, 589–608.
- 39N. Barbero, L. Napione, P. Quagliotto, S. Pavan, C. Barolo, E. Barni, F. Bussolino, G. Viscardi, Dyes Pigm. 2009, 83, 225–229.