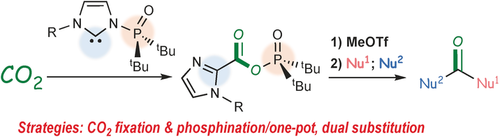
Strategic Utilization of Multifunctional Carbene for Direct Synthesis of Carboxylic–Phosphinic Mixed Anhydride from CO2
Graphical Abstract
Abstract
Direct synthesis of carboxylic–phosphinic mixed anhydrides has been achieved by treating carbon dioxide with N-phosphine oxide-substituted imidazolylidenes (PoxIms) that contain both nucleophilic carbene and electrophilic phosphorus moieties. This novel mixed anhydride was efficiently derivatized into an ester, an amide, and an unsymmetrical ketone via transformation into its corresponding imidazolium salt followed by a dual substitution reaction. The presented work used well-designed multifunctional carbene reagents to establish a novel utility for carbon dioxide in organic synthesis.
Mixed anhydrides comprising carboxylic and phosphinic acids are frequently employed for the preparation of carbonyl compounds such as carbamates and peptides.1 In particular, mixed anhydrides could be of great utility in organic synthesis when three conditions exist: when the compounds are stable towards thermal disproportionation, when they can be reacted with external nucleophiles specifically at the carbon center of the carbonyl moiety, and when they are isolable as shelf-stable solids.1d These anhydrides are prepared in the presence of a suitable base by either the reaction of a carboxylic acid with a phosphinic chloride or the reaction of an activated carboxylic acid derivative with a phosphinic acid; however, the concomitant production of a significant amount of salt is inevitable. Furthermore, carboxylic acid derivatives can be prepared from carbon dioxide.2 Thus, the direct synthesis of carboxylic–phosphinic mixed anhydrides from carbon dioxide is attractive from the aspects of step- and atom-economy.3
Recently, we reported the synthesis of N-phosphine oxide-substituted imidazolylidenes (PoxIms) as a novel class of isolable N-heterocyclic carbenes (NHCs) (Scheme 1).4, 5 PoxIms are equipped with two distinct Lewis bases, phosphine oxide and carbene, that led to the formation of either classical phosphine oxide–borane at −90 °C or carbene–borane adducts at room temperature (Scheme 1 a). The later adduct was used as a precursor for frustrated Lewis pairs6 comprising carbene and borane.4 On the other hand, the phosphine oxide moiety can also function as an electrophile (Scheme 1 b). Given this intriguing multifunctionality, we envisioned that the reaction of PoxIms with carbon dioxide could give an imidazolium-2-carboxylate followed by the nucleophilic addition of the carboxylate to the phosphine oxide to directly yield a novel type of carboxylic–phosphinic mixed anhydride. The formation of imidazolium-2-carboxylates by the reaction of NHCs and carbon dioxide was studied extensively.7 Furthermore, the imidazole moiety in the expected mixed anhydride can be easily transformed into an imidazolium moiety, which can be applied to the preparation of a variety of carbonyl compounds via dual substitution processes. Herein, we describe the use of strategically designed multifunctional carbenes as powerful tools for carbon dioxide transformation.
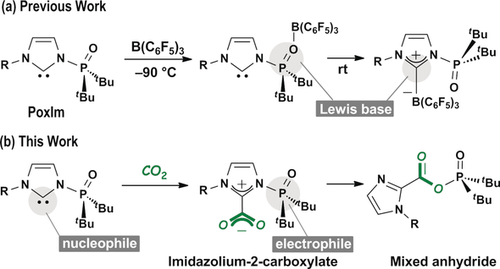
PoxIm plays a role of a) two distinct Lewis bases (previous work) and b) both a nucleophile and an electrophile (this work).
Treatment of PoxIm 1 a with carbon dioxide (5 atm) in toluene resulted in the formation of mixed anhydride 2 a, which was isolated in >99 % yield (Scheme 2). This reaction also proceeded quantitatively under 1 atm of carbon dioxide. The molecular structure of 2 a was identified by NMR spectroscopy and X-ray crystallography (Figure S6 in the Supporting Information), clearly showing the fixation of carbon dioxide and a migration of the phosphine oxide moiety to the carboxylic portion.8 In the 13C NMR spectrum, the resonances of C1 and C2 are observed at δC 137.8 (2JC,P=4.0 Hz) and 153.0 ppm (3JC,P=10.0 Hz), respectively, both of which appeared with the simultaneous disappearance of the resonance of the carbene carbon (δC 221.6 ppm, 2JC,P=26.0 Hz). In the 31P NMR spectrum, the resonance of the phosphinate moiety is observed at δP 69.2 ppm, following a downfield shift via the migration of the phosphorus center (1 a: δP 61.4 ppm). PoxIms 1 b–e also reacted with carbon dioxide to give the corresponding mixed anhydride 2 b–e, all of which were isolated as solids in excellent yields. The isolated solids of 2 b–e can be stored in the presence of air and moisture (20–30 °C, 30–50 % humidity) for at least 2 days without significant decomposition. Furthermore, no disproportionation was observed under ambient conditions in C6D6 for at least 21 days, which was confirmed using 2 b. Thus, these mixed anhydrides are ideal reagents for transfer of the carbonyl group. In fact, 2 b was converted into N-isopropyl imidazole derivatives efficiently (see the Supporting Information for details). N-Phosphine oxide-substituted imidazolinylidene (SPoxIm) 1 f gradually reacted with carbon dioxide at room temperature to afford 2 f in 76 % yield after 11 h. Heating promoted the reaction, and afforded 2 f in >99 % yield after 1 h at 80 °C.
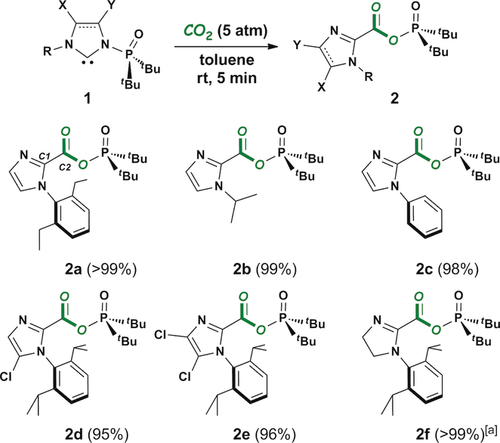
Reactions of PoxIms 1 a–e and SPoxIm 1 f with CO2. Isolated yields are given in parenthesis. [a] Reaction at 80 °C for 1 h.
PoxIm 1 g containing bis-N-phosphine oxide groups did not react with carbon dioxide under the ambient conditions, although 1 g was totally consumed in toluene at 80 °C after 1 h (Scheme 3). The quantitative formation of 2 g was confirmed when the product was dissolved in CD3CN at 22 °C; however, a mixture of 2 g and 2 g′ was given in yields of 58 and 42 %, respectively, after changing the solvent from CD3CN to C6D6 (Figure S8–S10). The formation of 2 g′, which was generated via the intermolecular addition of imidazole nitrogen to N-phosphine oxide in another 2 g, was expected according to NMR analyses in C6D6 (showing the resonances attributed to the imidazolium moiety) as well as to ESI-MS analysis (2 g′ of n=2 was detected).8 Replacement of the solvent from C6D6 to CD3CN again afforded 2 g as the sole product in >99 yield, which indicates that the intermolecular addition giving 2 g′ is reversible. Treatment of a mixture of 2 g and 2 g′ in C6D6 with B(C6F5)3 (1.0 equiv) resulted in the quantitative formation of 2 g⋅B(C6F5)3 as a sole product (Scheme 3), which also shows that the imidazole nitrogen in 2 g would be key for the generation of 2 g′.
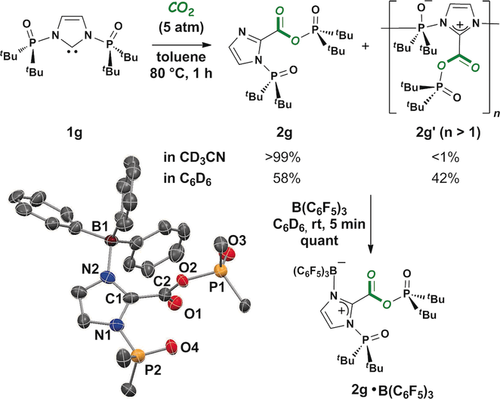
Reaction of 1 g with CO2, giving 2 g and 2 g′. Yields were determined by 31P NMR analysis at 22 °C. Molecular structure of 2 g⋅B(C6F5)3 is shown with ellipsoids set at 30 % probability. H, F (on C6F5), CH3 (on tBu), and solvated C6H6 molecules are omitted for clarity.
The formation of an imidazolium-2-carboxylate intermediate (A) was confirmed in CD2Cl2 at −90 °C when the reaction of 1 b with carbon dioxide (1 atm) was monitored by NMR spectroscopy (Scheme 4). A 16 % yield of 2 b was also detected when the reaction mixture was cooled to −90 °C in the NMR apparatus. An anti-orientation is proposed for the phosphine oxide moiety against the C1−C2 bond based on the results of theoretical studies (see below). The conversion of A into 2 b was observed even at 0 °C, and the quantitative formation of 2 b was confirmed when the reaction mixture was allowed to warm to room temperature, suggesting the rate-limiting step includes an irreversible transformation from A to 2 b.
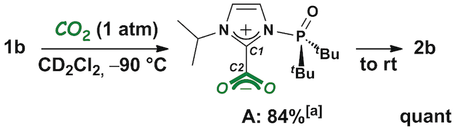
The formation of A and its conversion into 2 b. Yields are determined by NMR analyses. [a] 2 b was also formed in 16 % yield.
Plausible reaction mechanisms are further evaluated by DFT calculation at the B3LYP/6-31G+(d) level of theory (in toluene, at 25 °C, Figure 1 a), and 2 c was employed as a model substrate.8 Two conformers, aPxm and sPxm, are found depending on the orientation of the phosphine oxide moiety with respect to the lone pair of the carbene. The relative energy of sPxm is higher than that of aPxm by +5.5 kcal mol−1. The theoretical activation energy of the rotation of the phosphine oxide is +12.5 kcal mol−1, indicating its facile occurrence at 25 °C.4 While it was difficult to form the classical carbene–borane adduct using B(C6F5)3 and the anti-conformer PoxIm with N-aryl groups due to the steric repulsion between them, the formation of aPxm-CO2 (+5.2 kcal mol−1) by the reaction of aPxm with CO2 is expected to proceed more easily than the formation of sPxm-CO2 (+10.8 kcal mol−1). The latter takes place via the transformation of aPxm to sPxm followed by the reaction with CO2. TS2b (+15.7 kcal mol−1) is a more stable activation complex than TS2a (+19.8 kcal mol−1). The optimized structure of TS2b is given in Figure 1 b, where the carbene is approaching CO2 (C1⋅⋅⋅C2=2.210 Å) and the angle between the planes of the imidazolylidene ring and CO2 is out of perpendicular (67.5°). A similar plane angle of 71.4° is observed in the optimized structure of aPxm-CO2 (Figure 1 c), which is significantly smaller than the corresponding plane angle of 89.0° found in the IPr–CO2 adduct,7c but larger than the angles in the imidazolium-2-carboxylate with bis-N-methyl groups (29.1°).7b The bond length of C1−C2 in aPxm-CO2 is 1.550 Å, which is comparable with those found in other reported NHC–CO2 adducts showing experimental results of 1.52–1.54 Å.7 A slight elongation of the C1−C2 bond and an increment of the plane angle are found in sPxm-CO2 (1.557 Å and 86.6°, Figure 1 e) compared with those in aPxm-CO2. Although the formation of sPxm-CO2 might also be possible under the reaction conditions, sPxm-CO2 is expected to undergo rapid decarboxylation and eventually give aPxm-CO2. A direct transformation of aPxm-CO2 to sPxm-CO2 via TS3 (+25.2 kcal mol−1) would hardly occur since the theoretical activation barrier for this step is much larger than that for the migration of the phosphine oxide to give mxd ahd via TS4b (+21.0 kcal mol−1). Theoretical prediction for the formation of TS4b suggests that cleavage of the imidazolium–phosphine oxide bond (N2−P) and formation of the carboxylate–phosphine oxide bond (O2−P) occur in a concerted manner.9 The plane angle between the imidazolium and the carboxylate moieties in TS4b becomes 5.0° with interatomic distances of N2⋅⋅⋅P and P⋅⋅⋅O2 of 2.144 and 2.045 Å, respectively (Figure 1 d). The P atom adopts a distorted trigonal bipyramidal geometry with O2 and O3 atoms at the axial position. These calculation results are consistent with experimental observation, which means that the pathways affording mxd ahd via TS4b, in which overall activation energy is +21.0 kcal mol−1, are thus likely.
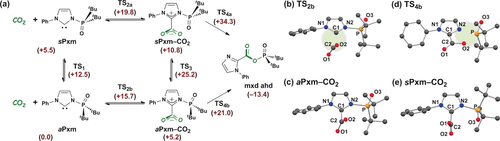
a) Plausible mechanisms for the production of the mixed anhydride (mxd ahd). The anti and syn conformers for PoxIm are denoted as aPxm and sPxm, respectively. Relative Gibbs free energies (kcal mol−1) in toluene (SMD implicit solvation model) with respect to [CO2+aPxm] (=+0.0) are shown, calculated by DFT at the B3LYP/6-31G+(d) level of theory (298.15 K). Optimized structure of b) TS2b, c) aPxm-CO2, d) TS4b and e) sPxm-CO2 are also given. Hydrogen atoms are omitted for clarity. Selected distances [Å] and angles [°]: b) C1⋅⋅⋅C2 2.210, C2–O1(O2) 1.193, N2–P 1.795; O1-C2-O2 152.5, C1-N2-P-O3 174.0, c) C1–C2 1.550, C2–O1(O2) 1.245–1.249, N2–P 1.845, O3–P 1.505; O1-C2-O2 132.3, C1-N2-P-O3 177.5, d) N2⋅⋅⋅P 2.144, O2⋅⋅⋅P 2.045, C1–C2 1.491, C2–O1 1.227, C2–O2 1.302, O3–P 1.518; O2-P-O3 151.4, O1-C2-O2 126.6, e) C1–C2 1.557, C2–O1(O2) 1.243–1.245, N2–P 1.822; O1-C2-O2 133.6, C1-N2-P-O3 10.0.
Treatment of 2 b (3.40 g, 10.82 mmol) with an equimolar amount of MeOTf in CH2Cl2 (5 mL) gave imidazolium salt 3 b (5.17 g, 10.81 mmol), which was isolated in 99 % yield as a shelf-stable solid under a dried nitrogen atmosphere (Scheme 5 a). The two potential leaving groups in 3 b are the imidazolium and the phosphinate moieties, which makes it a useful synthetic precursor for a variety of carbonyl compounds. In fact, dual substitution reactions of 3 b with two different nucleophiles in a one-pot manner gave ester 5 (1st; ZnAr2: 2nd; MeOH) and amide 6 (1st; ZnAr2: 2nd; CyNH2) in excellent yields, where Ar is p-OMeC6H4 (Scheme 5 b). In situ formation of 4 b and the zinc phosphinate salt by the reaction of 3 b and ZnAr2 (1.0 equiv) was directly observed by NMR analyses (Figures S17, S18). Furthermore, 3 b was utilized as a novel precursor for the preparation of unsymmetrical ketones (Scheme 5 c). Efficient synthetic method for unsymmetrical ketones is particularly of high utility when it can be carried out in a one-pot and straightforward manner.10 The reaction of 4 b generated in situ with NaCH2NO2 and NaCH(CO2Et)2 in DMF afforded unsymmetrical ketones 7 and 8 in 90 and 93 % isolated yield, respectively.11 Synthesis of unsymmetrical diaryl ketone was also examined to give 9 in 36 % isolated yield by utilizing two equivalents of ZnAr2 at the second substitution step. It should be noted that the present synthetic method for unsymmetrical ketones was conducted at mild temperature without tedious experimental procedures such as strict regulation of the reaction temperature and the concentration of nucleophiles. Thus, preliminary results shown herein ensure the promising reactivity of 3 b as a precursor for unsymmetrical ketones. The key for these dual substitution is utilizing arylzinc nucleophiles in the first substitution step in order to avoid an overreaction yielding a ketone and/or a tertiary alcohol given by aryl lithium and magnesium reagents.8
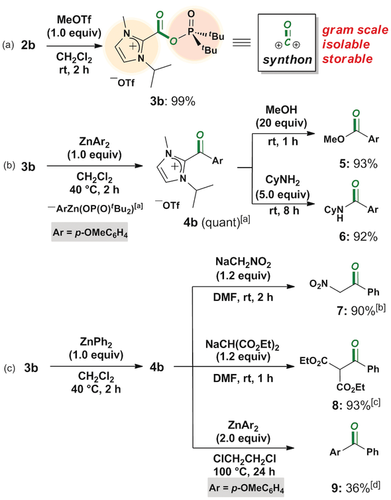
Synthetic applications of mixed anhydride 2 b: a) synthesis of imidazolium salt 3 b and b,c) its one-pot derivatization. 4 b was not isolated in the dual substitution reactions. Isolated yields are given. [a] NMR yield. [b] A mixture of keto/enol (8:1) isomers. [c] A mixture of keto/enol (5.9:1) isomers. [d] 1,2-Dichloroethane was used through dual substitutions.
A one-pot transformation of CO2 proceeding via key intermediate 3 b was conducted to afford 8 in 75 % isolated yield (Scheme 6), which also manifests this straightforward transformation processes of carbon dioxide toward unsymmetrical ketones.
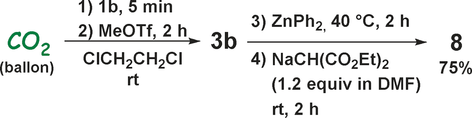
A thorough one-pot synthesis of 8 from CO2. Isolated yield of a mixture of keto/enol (8:1) isomers is given.
In summary, a synthetic route affording a novel carboxylic–phosphinic mixed anhydride directly from carbon dioxide has been achieved by using N-phosphine oxide substituted imidazolylidenes (PoxIms). The obtained carboxylic–phosphinic mixed anhydrides were utilized in the preparation of a variety of carbonyl compounds. A thorough one-pot transformation of CO2 into an unsymmetrical ketone is also demonstrated. Remarkably, PoxIm played dual key roles. It was nucleophile in the activation of carbon dioxide, and it also acted as an electrophile to transfer the phosphine oxide moiety to the carboxylic portion. The present work shows that strategically designed multifunctional carbene reagents offer a novel utility for carbon dioxide in organic synthesis.
Acknowledgements
This work was supported by Grants-in Aid for Young Scientists (A) (JSPS KAKENHI Grant Number JP25708018), Encouragement for Young Scientists (B) (JSPS KAKENHI Grant Number JP15K17824), and Scientific Research on Innovative Areas “Stimuli-responsive Chemical Species (No. 2408)” (JSPS KAKENHI Grant Number JP15H00943) and “Precisely Designed Catalysts with Customized Scaffolding (JSPS KAKENHI Grant Number JP15H05803)”. Y.H. acknowledges support from the Frontier Research Base for Global Young Researchers, Osaka University, on the program of MEXT.