Nucleophilic Transfer Reactions of the [Si(C2F5)3]− Moiety
Nico Schwarze
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorDr. Simon Steinhauer
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorBeate Neumann
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorDr. Hans-Georg Stammler
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Berthold Hoge
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorNico Schwarze
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorDr. Simon Steinhauer
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorBeate Neumann
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorDr. Hans-Georg Stammler
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Berthold Hoge
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorGraphical Abstract
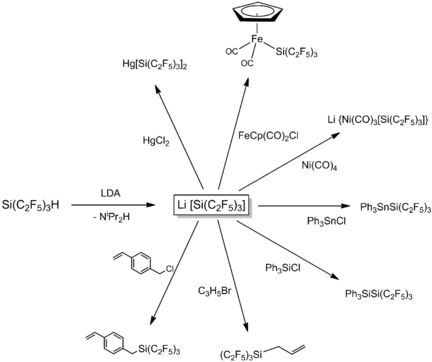
Easily transferred: The tris(pentafluoroethyl)silanide anion is accessible by the deprotonation of Si(C2F5)3H at low temperatures. Subsequent quenching with suitable electrophiles leads to a plethora of tris(pentafluoroethyl)silane derivatives and underlines the versatility of Li[Si(C2F5)3] as a powerful nucleophilic transfer reagent of the [Si(C2F5)3]− unit.
Abstract
The tris(pentafluoroethyl)silanide anion is accessible by the deprotonation of Si(C2F5)3H at low temperatures. Subsequent quenching of the resulting salt-like compounds with suitable electrophiles, such as transition-metal complexes or Group 14 element halides, leads to a plethora of novel tris(pentafluoroethyl)silane derivatives. This underlines the versatility of Li[Si(C2F5)3] as a powerful nucleophilic transfer reagent.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201609575-sup-0001-misc_information.pdf321.3 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1G. K. S. Prakash, A. K. Yudin, Chem. Rev. 1997, 97, 757–786.
- 2
- 2aI. Ruppert, K. Schlich, W. Volbach, Tetrahedron Lett. 1984, 25, 2195–2198;
- 2bG. K. S. Prakash, M. Mandal, J. Fluorine Chem. 2001, 112, 123–131;
- 2cJ. Wiedemann, T. Heinzer, G. Mloston, G. K. S. Prakash, G. A. Olah, Angew. Chem. Int. Ed. 1998, 37, 820–821;
10.1002/(SICI)1521-3773(19980403)37:6<820::AID-ANIE820>3.0.CO;2-M CAS PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 880–881;
- 2dA. Olejniczak, A. Katrusiak, A. Vij, J. Fluorine Chem. 2008, 129, 1090–1095.
- 3H. Beckers, Dissertation, Bergische Universität Wuppertal—Gesamthochschule, Wuppertal, 1987.
- 4K. G. Sharp, T. D. Coyle, Inorg. Chem. 1972, 11, 1259–1264.
- 5G. K. S. Prakash, P. V. Jog, P. T. D. Batamack, G. A. Olah, Science 2012, 338, 1324–1327.
- 6K. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881.
- 7S. Steinhauer, J. Bader, H.-G. Stammler, N. Ignat‘ev, B. Hoge, Angew. Chem. Int. Ed. 2014, 53, 5206–5209; Angew. Chem. 2014, 126, 5307–5310.
- 8
- 8aS. Steinhauer, H.-G. Stammler, B. Neumann, N. Ignat‘ev, B. Hoge, Angew. Chem. Int. Ed. 2014, 53, 562–564; Angew. Chem. 2014, 126, 573–575;
- 8bN. Ignat'ev, M. Schulte, B. Hoge, S. Steinhauer (Merck Patent GmbH), DE 102012006896 A1, 2012;
- 8cB. Hoge, N. Ignat'ev, M. Schulte, S. Steinhauer (BASF SE), WO 2013\150448 A1, 2013;
- 8dS. Steinhauer, T. Böttcher, N. Schwarze, B. Neumann, H.-G. Stammler, B. Hoge, Angew. Chem. Int. Ed. 2014, 53, 13269–13272; Angew. Chem. 2014, 126, 13485–13488.
- 9
- 9aB. Waerder, S. Steinhauer, B. Neumann, H.-G. Stammler, A. Mix, Y. V. Vishnevskiy, B. Hoge, N. Mitzel, Angew. Chem. Int. Ed. 2014, 53, 11640–11644; Angew. Chem. 2014, 126, 11824–11828;
- 9bM. Heinrich, A. Marhold, A. Kolomeitsev, A. Kadyrov, G.-V. Röschenthaler, J. Barten, DE10128703A1, 2001;
- 9cM. H. Königsmann, PhD Thesis, Universität Bremen, 2005.
- 10B. Waerder, S. Steinhauer, J. Bader, B. Neumann, H.-G. Stammler, Y. V. Vishnevskiy, B. Hoge, N. W. Mitzel, Dalton Trans. 2015, 44, 13347–13358.
- 11N. Schwarze, S. Steinhauer, B. Neumann, H.-G. Stammler, B. Hoge, Angew. Chem. Int. Ed. 2016, 55, 16156–16160; Angew. Chem. 2016, 128, 16390–16394.
- 12J. March, Advanced Organic Chemistry, 4th ed., Wiley, New York, 1992, pp. 369–500.
- 13U. Schubert, G. Kraft, E. Walther, Z. Anorg. Allg. Chem. 1984, 519, 96–106.
- 14
- 14aC. A. Tolman, J. Am. Chem. Soc. 1970, 92, 2956–2965;
- 14bC. A. Tolman, W. C. Seidel, L. W. Gosser, J. Am. Chem. Soc. 1974, 96, 53–60;
- 14cC. A. Tolman, Chem. Rev. 1977, 77, 313–348.
- 15R. H. Crabtree, Carbonyls, Phosphine Complexes, and Ligand Substitution Reactions, The Organometallic Chemistry of the Transition Metals, 4th ed., Wiley, Hoboken, 2005, pp. 87–124.
10.1002/0471718769.ch4 Google Scholar
- 16J. B. Tice, A. V. G. Chizmeshya, T. L. Groy, J. Kouvetakis, Inorg. Chem. 2009, 48, 6314–6320.
- 17S. Adams, M. Dräger, J. Organomet. Chem. 1987, 323, 11–20.
- 18N. Wiberg, H. Schuster, A. Simon, K. Peters, Angew. Chem. Int. Ed. Engl. 1986, 25, 79–80; Angew. Chem. 1986, 98, 100–101.
- 19O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann, J. Appl. Crystallogr. 2009, 42, 339–341.
- 20G. M. Sheldrick, Acta Crystallogr. Sect. A 2008, 64, 112–122.