Gram-Scale Synthesis of Chiral Cyclopropane-Containing Drugs and Drug Precursors with Engineered Myoglobin Catalysts Featuring Complementary Stereoselectivity
Dr. Priyanka Bajaj
Department of Chemistry, University of Rochester, 120 Trustee Road, Rochester, NY, 14627 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Gopeekrishnan Sreenilayam
Department of Chemistry, University of Rochester, 120 Trustee Road, Rochester, NY, 14627 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Vikas Tyagi
Department of Chemistry, University of Rochester, 120 Trustee Road, Rochester, NY, 14627 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Rudi Fasan
Department of Chemistry, University of Rochester, 120 Trustee Road, Rochester, NY, 14627 USA
Search for more papers by this authorDr. Priyanka Bajaj
Department of Chemistry, University of Rochester, 120 Trustee Road, Rochester, NY, 14627 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Gopeekrishnan Sreenilayam
Department of Chemistry, University of Rochester, 120 Trustee Road, Rochester, NY, 14627 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Vikas Tyagi
Department of Chemistry, University of Rochester, 120 Trustee Road, Rochester, NY, 14627 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Rudi Fasan
Department of Chemistry, University of Rochester, 120 Trustee Road, Rochester, NY, 14627 USA
Search for more papers by this authorGraphical Abstract
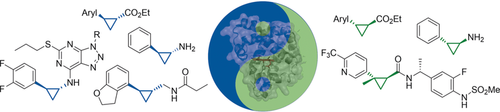
Chiral cyclopropanes à la carte: Myoglobin-based cyclopropanation catalysts featuring complementary stereoselectivity were developed for the synthesis of 1-carboxy-2-aryl-cyclopropanes. The engineered hemoproteins were applied in whole-cell reactions to afford cyclopropane-containing drugs and precursors thereof at the gram scale, in high yield, and with excellent diastereo- and enantioselectivity.
Abstract
Engineered hemoproteins have recently emerged as promising systems for promoting asymmetric cyclopropanations, but variants featuring predictable, complementary stereoselectivity in these reactions have remained elusive. In this study, a rationally driven strategy was implemented and applied to engineer myoglobin variants capable of providing access to 1-carboxy-2-aryl-cyclopropanes with high trans-(1R,2R) selectivity and catalytic activity. The stereoselectivity of these cyclopropanation biocatalysts complements that of trans-(1S,2S)-selective variants developed here and previously. In combination with whole-cell biotransformations, these stereocomplementary biocatalysts enabled the multigram synthesis of the chiral cyclopropane core of four drugs (Tranylcypromine, Tasimelteon, Ticagrelor, and a TRPV1 inhibitor) in high yield and with excellent diastereo- and enantioselectivity (98–99.9% de; 96–99.9% ee). These biocatalytic strategies outperform currently available methods to produce these drugs.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201608680-sup-0001-misc_information.pdf4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1T. T. Talele, J. Med. Chem. 2016, 59, 8712–8756.
- 2
- 2aM. P. Doyle, D. C. Forbes, Chem. Rev. 1998, 98, 911–936;
- 2bH. Lebel, J. F. Marcoux, C. Molinaro, A. B. Charette, Chem. Rev. 2003, 103, 977–1050;
- 2cH. Pellissier, Tetrahedron 2008, 64, 7041–7095;
- 2dR. E. Lowenthal, A. Abiko, S. Masamune, Tetrahedron Lett. 1990, 31, 6005–6008; for representative examples with acceptor-only diazo reagents, see:
- 2eD. A. Evans, K. A. Woerpel, M. M. Hinman, M. M. Faul, J. Am. Chem. Soc. 1991, 113, 726–728;
- 2fM. P. Doyle, W. R. Winchester, J. A. A. Hoorn, V. Lynch, S. H. Simonsen, R. Ghosh, J. Am. Chem. Soc. 1993, 115, 9968–9978;
- 2gH. Nishiyama, Y. Itoh, H. Matsumoto, S. B. Park, K. Itoh, J. Am. Chem. Soc. 1994, 116, 2223–2224;
- 2hT. Uchida, R. Irie, T. Katsuki, Synlett 1999, 1163–1165;
- 2iC. M. Che, J. S. Huang, F. W. Lee, Y. Li, T. S. Lai, H. L. Kwong, P. F. Teng, W. S. Lee, W. C. Lo, S. M. Peng, Z. Y. Zhou, J. Am. Chem. Soc. 2001, 123, 4119–4129;
- 2jL. Y. Huang, Y. Chen, G. Y. Gao, X. P. Zhang, J. Org. Chem. 2003, 68, 8179–8184;
- 2kS. Fantauzzi, E. Gallo, E. Rose, N. Raoul, A. Caselli, S. Issa, F. Ragaini, S. Cenini, Organometallics 2008, 27, 6143–6151;
- 2lD. M. Carminati, D. Intrieri, A. Caselli, S. Le Gac, B. Boitrel, L. Toma, L. Legnani, E. Gallo, Chem. Eur. J. 2016, 22, 13599–13612.
- 3
- 3aP. S. Coelho, E. M. Brustad, A. Kannan, F. H. Arnold, Science 2013, 339, 307–310;
- 3bP. S. Coelho, Z. J. Wang, M. E. Ener, S. A. Baril, A. Kannan, F. H. Arnold, E. M. Brustad, Nat. Chem. Biol. 2013, 9, 485–487.
- 4M. Bordeaux, V. Tyagi, R. Fasan, Angew. Chem. Int. Ed. 2015, 54, 1744–1748; Angew. Chem. 2015, 127, 1764–1768.
- 5J. G. Gober, A. E. Rydeen, E. J. Gibson-O'Grady, J. B. Leuthaeuser, J. S. Fetrow, E. M. Brustad, ChemBioChem 2016, 17, 394–397.
- 6
- 6aQ. Wu, P. Soni, M. T. Reetz, J. Am. Chem. Soc. 2013, 135, 1872–1881;
- 6bA. Z. Walton, B. Sullivan, A. C. Patterson-Orazem, J. D. Stewart, ACS Catal. 2014, 4, 2307–2318;
- 6cP. N. Scheller, S. Fademrecht, S. Hofelzer, J. Pleiss, F. Leipold, N. J. Turner, B. M. Nestl, B. Hauer, ChemBioChem 2014, 15, 2201–2204;
- 6dJ. Y. van der Meer, H. Poddar, B. J. Baas, Y. F. Miao, M. Rahimi, A. Kunzendorf, R. van Merkerk, P. G. Tepper, E. M. Geertsema, A. M. W. H. Thunnissen, W. J. Quax, G. J. Poelarends, Nat. Commun. 2016, 7, 10911.
- 7P. F. Mugford, U. G. Wagner, Y. Jiang, K. Faber, R. J. Kazlauskas, Angew. Chem. Int. Ed. 2008, 47, 8782–8793; Angew. Chem. 2008, 120, 8912–8923.
- 8
- 8aZ. J. Wang, H. Renata, N. E. Peck, C. C. Farwell, P. S. Coelho, F. H. Arnold, Angew. Chem. Int. Ed. 2014, 53, 6810–6813; Angew. Chem. 2014, 126, 6928–6931;
- 8bH. Renata, Z. J. Wang, R. Z. Kitto, F. H. Arnold, Catal. Sci. Technol. 2014, 4, 3640–3643;
- 8cP. Srivastava, H. Yang, K. Ellis-Guardiola, J. C. Lewis, Nat. Commun. 2015, 6, 7789.
- 9
- 9aG. Sreenilayam, R. Fasan, Chem. Commun. 2015, 51, 1532–1534;
- 9bV. Tyagi, R. B. Bonn, R. Fasan, Chem. Sci. 2015, 6, 2488–2494;
- 9cV. Tyagi, R. Fasan, Angew. Chem. Int. Ed. 2016, 55, 2512–2516; Angew. Chem. 2016, 128, 2558–2562;
- 9dS. Giovani, R. Singh, R. Fasan, Chem. Sci. 2016, 7, 234–239.
- 10M. Bordeaux, R. Singh, R. Fasan, Bioorg. Med. Chem. 2014, 22, 5697–5704.
- 11
- 11aM. T. Lundemo, J. M. Woodley, Appl. Microbiol. Biotechnol. 2015, 99, 2465–2483;
- 11bR. J. Sowden, S. Yasmin, N. H. Rees, S. G. Bell, L. L. Wong, Org. Biomol. Chem. 2005, 3, 57–64;
- 11cR. Fasan, M. M. Chen, N. C. Crook, F. H. Arnold, Angew. Chem. Int. Ed. 2007, 46, 8414–8418; Angew. Chem. 2007, 119, 8566–8570;
- 11dR. Fasan, N. C. Crook, M. W. Peters, P. Meinhold, T. Buelter, M. Landwehr, P. C. Cirino, F. H. Arnold, Biotechnol. Bioeng. 2011, 108, 500–510;
- 11eM. Ringle, Y. Khatri, J. Zapp, F. Hannemann, R. Bernhardt, Appl. Microbiol. Biotechnol. 2013, 97, 7741–7754;
- 11fC. A. Müller, A. Dennig, T. Welters, T. Winkler, A. J. Ruff, W. Hummel, H. Groger, U. Schwaneberg, J. Biotechnol. 2014, 191, 196–204;
- 11gF. Tieves, I. N. Erenburg, O. Mahmoud, V. B. Urlacher, Biotechnol. Bioeng. 2016, 113, 1845–1852.
- 12A. Burger, W. L. Yost, J. Am. Chem. Soc. 1948, 70, 2198–2201.
- 13J. Catt, G. Johnson, D. Keavy, R. Mattson, M. Parker, K. Takaki, J. Yevich (BMS), U.S. Pat. 5981571, 1999.
- 14K. J. Butcher, S. M. Denton, S. E. Field, A. T. Gillmore, G. W. Harbottle, R. M. Howard, D. A. Laity, C. J. Ngono, B. A. Pibworth, Org. Process Res. Dev. 2011, 15, 1192–1200.
- 15B. Springthorpe et al., Bioorg. Med. Chem. Lett. 2007, 17, 6013–6018.
- 16T. N. Riley, C. G. Brier, J. Med. Chem. 1972, 15, 1187–1188.
- 17J. H. Simpson, J. Godfrey, R. Fox, A. Kotnis, D. Kacsur, J. Hamm, M. Totelben, V. Rosso, R. Mueller, E. Delaney, R. P. Deshpande, Tetrahedron: Asymmetry 2003, 14, 3569–3574.
- 18
- 18aH. Zhang, J. Liu, L. Zhang, L. Kong, H. Yao, H. Sun, Bioorg. Med. Chem. Lett. 2012, 22, 3598–3602;
- 18bS. Guile, D. Hardern, B. Springthorpe, P. Willis, WO/2000/034283, 2000;
- 18cA. Clark, E. Jones, U. Larsson, A. Minidis, WO/2001/092200, 2001;
- 18dK. G. Hugentobler, H. Sharif, M. Rasparini, R. S. Heath, N. J. Turner, Org. Biomol. Chem. 2016, 14, 8064–8067.
- 19H. M. Key, P. Dydio, D. S. Clark, J. F. Hartwig, Nature 2016, 534, 534–537.