Structure of Monomeric Transthyretin Carrying the Clinically Important T119M Mutation
Dr. Jin Hae Kim
Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), Von-Siebold-Strasse 3a, 37075 Göttingen, Germany
Search for more papers by this authorDr. Javier Oroz
Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), Von-Siebold-Strasse 3a, 37075 Göttingen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Markus Zweckstetter
Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), Von-Siebold-Strasse 3a, 37075 Göttingen, Germany
Max-Planck-Institut für Biophysikalische Chemie, Am Fassberg 11, 37077 Göttingen, Germany
Department of Neurology, University Medical Center Göttingen, University of Göttingen, Waldweg 33, 37073 Göttingen, Germany
Search for more papers by this authorDr. Jin Hae Kim
Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), Von-Siebold-Strasse 3a, 37075 Göttingen, Germany
Search for more papers by this authorDr. Javier Oroz
Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), Von-Siebold-Strasse 3a, 37075 Göttingen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Markus Zweckstetter
Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), Von-Siebold-Strasse 3a, 37075 Göttingen, Germany
Max-Planck-Institut für Biophysikalische Chemie, Am Fassberg 11, 37077 Göttingen, Germany
Department of Neurology, University Medical Center Göttingen, University of Göttingen, Waldweg 33, 37073 Göttingen, Germany
Search for more papers by this authorGraphical Abstract
Abstract
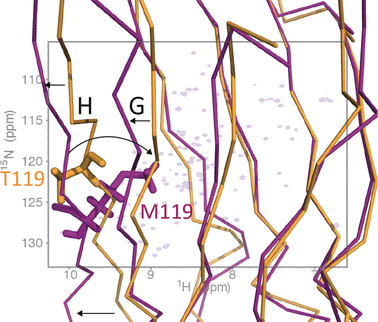
Mutations in the protein transthyretin can cause as well as protect individuals from transthyretin amyloidosis, an incurable fatal inherited disease. Little is known, however, about the structural basis of pathogenic and clinically protective transthyretin mutants. Here we determined the solution structure of a transthyretin monomer that carries the clinically important T119M mutation. The structure displays a non-native arrangement that is distinct from all known structures of transthyretin and highlights the importance of high-resolution studies in solution for understanding molecular processes that lead to amyloid diseases.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201608516-sup-0001-misc_information.pdf285.6 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. D. Benson, T. Uemichi, Amyloid-Int. J. Exp. Clin. Invest. 1996, 3, 44–56.
- 2L. H. Connors, A. Lim, T. Prokaeva, V. A. Roskens, C. E. Costello, Amyloid 2003, 10, 160–184.
- 3
- 3aC. C. Blake, M. J. Geisow, I. D. Swan, C. Rerat, B. Rerat, J. Mol. Biol. 1974, 88, 1–12;
- 3bS. M. Johnson, S. Connelly, C. Fearns, E. T. Powers, J. W. Kelly, J. Mol. Biol. 2012, 421, 185–203.
- 4A. Hörnberg, T. Eneqvist, A. Olofsson, E. Lundgren, A. E. Sauer-Eriksson, J. Mol. Biol. 2000, 302, 649–669.
- 5
- 5aP. Hammarstrom, F. Schneider, J. W. Kelly, Science 2001, 293, 2459–2462;
- 5bT. Coelho, M. Carvalho, M. J. Saraiva, I. Alves, M. R. Almeida, P. P. Costa, J. Rheumatol. 1993, 20, 179;
- 5cT. Coelho, R. Chorão, A. Sousa, I. Alves, M. F. Torres, M. J. M. Saraiva, Neuromusc. Disord. 1996, 6(Suppl 1), 20.
- 6P. Hammarstrom, X. Jiang, A. R. Hurshman, E. T. Powers, J. W. Kelly, Proc. Natl. Acad. Sci. USA 2002, 99 (Suppl 4), 16427–16432.
- 7K. Liu, J. W. Kelly, D. E. Wemmer, J. Mol. Biol. 2002, 320, 821–832.
- 8W. Colon, J. W. Kelly, Biochemistry 1992, 31, 8654–8660.
- 9A. Olofsson, H. J. Ippel, V. Baranov, P. Horstedt, S. Wijmenga, E. Lundgren, J. Biol. Chem. 2001, 276, 39592–39599.
- 10
- 10aA. D. Ferrão-Gonzales, S. O. Souto, J. L. Silva, D. Foguel, Proc. Natl. Acad. Sci. USA 2000, 97, 6445–6450;
- 10bA. D. Ferrão-Gonzales, L. Palmieri, M. Valory, J. L. Silva, H. Lashuel, J. W. Kelly, D. Foguel, J. Mol. Biol. 2003, 328, 963–974;
- 10cT. Eneqvist, K. Andersson, A. Olofsson, E. Lundgren, A. E. Sauer-Eriksson, Mol. Cell 2000, 6, 1207–1218;
- 10dJ. K. Das, S. S. Mall, A. Bej, S. Mukherjee, Angew. Chem. Int. Ed. 2014, 53, 12781–12784; Angew. Chem. 2014, 126, 12995–12998.
- 11M. P. Sebastião, V. Lamzin, M. J. Saraiva, A. M. Damas, J. Mol. Biol. 2001, 306, 733–744.
- 12X. Jiang, C. S. Smith, H. M. Petrassi, P. Hammarstrom, J. T. White, J. C. Sacchettini, J. W. Kelly, Biochemistry 2001, 40, 11442–11452.
- 13K. H. Lim, H. J. Dyson, J. W. Kelly, P. E. Wright, J. Mol. Biol. 2013, 425, 977–988.
- 14L. Saelices, L. M. Johnson, W. Y. Liang, M. R. Sawaya, D. Cascio, P. Ruchala, J. Whitelegge, L. Jiang, R. Riek, D. S. Eisenberg, J. Biol. Chem. 2015, 290, 28932.
- 15
- 15aN. Tjandra, A. Bax, Science 1997, 278, 1111–1114;
- 15bJ. R. Tolman, J. M. Flanagan, M. A. Kennedy, J. H. Prestegard, Nat. Struct. Biol. 1997, 4, 292–297.
- 16
- 16aJ. H. Prestegard, A. I. Kishore, Curr. Opin. Chem. Biol. 2001, 5, 584–590;
- 16bK. Chen, N. Tjandra, Top. Curr. Chem. 2012, 326, 47–67;
- 16cJ. R. Tolman, Curr. Opin. Struct. Biol. 2001, 11, 532–539.
- 17M. Zweckstetter, Nat. Protoc. 2008, 3, 679–690.
- 18
- 18aY. Shen, A. Bax, Methods Mol. Biol. 2015, 1260, 17–32;
- 18bM. V. Berjanskii, D. S. Wishart, J. Am. Chem. Soc. 2005, 127, 14970–14971.
- 19
- 19aK. H. Lim, A. K. Dasari, I. Hung, Z. Gan, J. W. Kelly, D. E. Wemmer, Biochemistry 2016, 55, 1941–1944;
- 19bM. Yang, M. Lei, R. Bruschweiler, S. Huo, Biophys. J. 2005, 89, 433–443.
- 20X. Jiang, J. N. Buxbaum, J. W. Kelly, Proc. Natl. Acad. Sci. USA 2001, 98, 14943–14948.
- 21
- 21aA. Bax, Curr. Opin. Struct. Biol. 1994, 4, 738–744;
- 21bM. Sattler, J. Schleucher, C. Griesinger, Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 93–158.
- 22A. Bax, G. M. Clore, A. M. Gronenborn, J. Magn. Reson. 1990, 88, 425–431.
- 23
- 23aK. Wuthrich, 1986;
- 23bD. Marion, P. C. Driscoll, L. E. Kay, P. T. Wingfield, A. Bax, A. M. Gronenborn, G. M. Clore, Biochemistry 1989, 28, 6150–6156.
- 24F. Delaglio, S. Grzesiek, G. W. Vuister, G. Zhu, J. Pfeifer, A. Bax, J. Biomol. NMR 1995, 6, 277–293.
- 25Y. Shen, A. Bax, J. Biomol. NMR 2013, 56, 227–241.
- 26L. Yao, J. Ying, A. Bax, J. Biomol. NMR 2009, 43, 161–170.
- 27P. Guntert, Methods Mol. Biol. 2004, 278, 353–378.
- 28A. Bhattacharya, R. Tejero, G. T. Montelione, Proteins Struct. Funct. Bioinf. 2007, 66, 778–795.