Tandem Difluoroalkylation-Arylation of Enamides Catalyzed by Nickel
Ji-Wei Gu
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
These authors contributed equally to this work.
Search for more papers by this authorQiao-Qiao Min
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
These authors contributed equally to this work.
Search for more papers by this authorLing-Chao Yu
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xingang Zhang
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorJi-Wei Gu
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
These authors contributed equally to this work.
Search for more papers by this authorQiao-Qiao Min
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
These authors contributed equally to this work.
Search for more papers by this authorLing-Chao Yu
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xingang Zhang
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorGraphical Abstract
Abstract
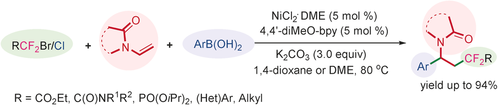
A nickel-catalyzed three-component reaction for the synthesis of difluoroalkylated compounds through tandem difluoroalkylation-arylation of enamides has been developed. The reaction tolerates a variety of arylboronic acids and widely available difluoroalkyl bromides, and even the relatively inert substrate chlorodifluoroacetate. The significant advantages of this protocol are the low-cost nickel catalyst, synthetic convenience, excellent functional-group compatibility and high reaction efficiency.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201606458-sup-0001-misc_information.pdf5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. Muller, C. Faeh, F. Diederich, Science 2007, 317, 1881;
- 1bD. O'Hagan, Chem. Soc. Rev. 2008, 37, 308;
- 1cC. M. Blackburn, D. A. England, F. Kolkmann, J. Chem. Soc. Chem. Commun. 1981, 930;
- 1dG. M. Blackburn, D. E. Kent, F. Kolkmann, J. Chem. Soc. Perkin Trans. 1 1984, 1119.
- 2
- 2aJ. O. Link, J. G. Taylor, L. Xu, M. Mitchell, H. Guo, H. Liu, D. Kato, T. Kirschberg, J. Sun, N. Squires, J. Parrish, T. Keller, Z.-Y. Yang, C. Yang, M. Matles, Y. Wang, K. Wang, G. Cheng, Y. Tian, E. Mogalian, E. Mondou, M. Cornpropst, J. Perry, M. C. Desai, J. Med. Chem. 2014, 57, 2033;
- 2bF. Akahoshi, A. Ashimori, H. Sakashita, T. Yoshimura, M. Eda, T. Imada, M. Nakajima, N. Mitsutomi, S. Kuwahara, T. Ohtsuka, C. Fukaya, M. Miyazaki, N. Nakamura, J. Med. Chem. 2001, 44, 1297;
- 2cA. A. Makarov, P. O. Tsvetkov, C. Villard, D. Esquieu, B. Pourroy, J. Fahy, D. Braguer, V. Peyrot, D. Lafitte, Biochemistry 2007, 46, 14899.
- 3For reviews, see:
- 3aB. Chen, D. Vicic, Top. Organomet. Chem. 2014, 52, 113;
- 3bM.-C. Belhomme, T. Besset, T. Poisson, X. Pannecoucke, Chem. Eur. J. 2015, 21, 12836.
- 4Y.-L. Xiao, Q.-Q. Min, C. Xu, R.-W. Wang, X. Zhang, Angew. Chem. Int. Ed. 2016, 55, 5837; Angew. Chem. 2016, 128, 5931.
- 5For poineering studies on nickel-catalyzed cross-coupling of alkylhalides with aryl borons, see:
- 5aJ. Zhou, G. C. Fu, J. Am. Chem. Soc. 2004, 126, 1340;
- 5bA. Wilsily, F. Tramutola, N. A. Owston, G. C. Fu, J. Am. Chem. Soc. 2012, 134, 5794;
- 5cS. L. Zultanski, G. C. Fu, J. Am. Chem. Soc. 2013, 135, 624.
- 6For pioneering studies on trifluoromethyl nickel complexes, see:
- 6aG. G. Dubinina, W. W. Brennessel, J. L. Miller, D. A. Vicic, Organometallics 2008, 27, 3933;
- 6bA. Klein, D. A. Vicic, C. Biewer, I. Kieltsch, K. Stirnat, C. Hamacher, Organometallics 2012, 31, 5334;
- 6cC.-P. Zhang, H. Wang, A. Klein, C. Biewer, K. Stirnat, Y. Yamaguchi, L. Xu, V. Gomez-Benitez, D. A. Vicic, J. Am. Chem. Soc. 2013, 135, 8141.
- 7For nickel-catalyzed cross-coupling reactions of difluoroalkylations, see:
- 7aY.-L. Xiao, W.-H. Guo, G.-Z. He, Q. Pan, X. Zhang, Angew. Chem. Int. Ed. 2014, 53, 9909; Angew. Chem. 2014, 126, 10067;
- 7bY.-L. Xiao, Q. Pan, X. Zhang, Acta Chim. Sin. 2015, 73, 383;
- 7cL. Xu, D. A. Vicic, J. Am. Chem. Soc. 2016, 138, 2536, and Ref. [4].
- 8A synthesis of difluoroalkylated compounds through palladium-catalyzed aryldifluoroacetylation of alkynes has also been reported, but the noble metal and expensive iododifluoroacetate (ICF2CO2Et) were used. In addition, only alkynes were suitable substrates in these reactions, and the reaction of alkenes remains a synthetic challenge. See:
- 8aY.-T. He, Q. Wang, L.-H. Li, X.-Y. Liu, P.-F. Xu, Y.-M. Liang, Org. Lett. 2015, 17, 5188;
- 8bZ. Li, A. Garcia-Dominguez, C. Nevado, J. Am. Chem. Soc. 2015, 137, 11610.
- 9During our manuscript preparation, a nickel-catalyzed three-component conjunctive cross-coupling was been reported: T. Qin, J. Cornella, C. Li, L. R. Malins, J. T. Edwards, S. Kawamura, B. D. Maxwell, M. D. Eastgate, P. S. Baran, Science 2016, 352, 801.
- 10For mechanistic studies on nickel-catalyzed cross-coupling with unactivated alkyl halides, see:
- 10aG. D. Jones, J. L. Martin, C. McFarland, O. R. Allen, R. E. Hall, A. D. Haley, R. J. Brandon, T. Konovalova, P. J. Desrochers, P. Pulay, D. A. Vicic, J. Am. Chem. Soc. 2006, 128, 13175;
- 10bX. Hu, Chem. Sci. 2011, 2, 1867.
- 11
- 11aD. A. Kendrick, C. Danzin, M. Kolb, J. Med. Chem. 1989, 32, 170;
- 11bD. W. Konas, J. K. Coward, Org. Lett. 1999, 1, 2105;
- 11cS. Bogen, A. Arasappan, W. P. S. Ruan, A. K. Saksena, V. Girijavallabhan, F. G. Njoroge, Bioorg. Med. Chem. Lett. 2008, 18, 4219;
- 11dL. Leung, C. Tomassi, K. V. Beneden, T. Decruy, D. Elewaut, T. Elliott, A. Al-Shamkhani, C. Ottensmeier, S. V. Calenbergh, J. Werner, T. Williams, B. Linclau, Org. Lett. 2008, 10, 4433.
- 12
- 12aW. R. Dolbier, Chem. Rev. 1996, 96, 1557;
- 12bN. Zhang, S. R. Samanta, B. M. Rosen, V. Percec, Chem. Rev. 2014, 114, 5848.
- 13
- 13aT. Yajima, K. Yamaguchi, R. Hirokane, E. Nogami, J. Fluorine Chem. 2013, 150, 1;
- 13bT. Sifferlen, A. Boller, A. Chardonneau, E. Cottreel, J. Gatfield, A. Treiber, C. Roch, F. Jenck, H. Aissaoui, J. T. Williams, C. Brotschi, B. Heidmann, R. Siegrist, C. Boss, Bioorg. Med. Chem. Lett. 2015, 25, 1884;
- 13cH. Aissaoui, C. Boss, C. Brotschi, B. Heidmann, T. Sifferlen, J. T. Williams, WO 2012063207 A1, 2012.
- 14H. Cong, G. C. Fu, J. Am. Chem. Soc. 2014, 136, 3788.
- 15J. E. Baldwin, Chem. Rev. 2003, 103, 1197.