Aminopyridinyl–Pseudodeoxycytidine Derivatives Selectively Stabilize Antiparallel Triplex DNA with Multiple CG Inversion Sites
Graphical Abstract
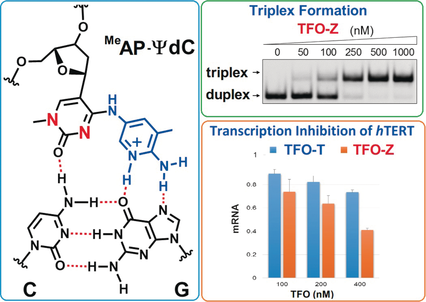
Pseudo-deoxycytidine (ΨdC) derivatives were developed for the selective recognition of the CG base pair to expand the triplex-forming sequence. The ΨdCs formed a stable triplex with the promoter of the hTERT gene containing four CG inversion sites, and effectively inhibited its transcription in human cancer cells, and may lead to the development of new triplex-forming oligonucleotides.
Abstract
The sequence-specific formation of triplex DNA offers a potential basis for genome-targeting technologies. In an antiparallel triplex DNA, the sequence-specificity is established by the formation of specific base triplets (G−GC, A−AT, and T−AT) between a triplex-forming oligonucleotide (TFO) and a duplex DNA. However, there are no natural nucleosides that can selectively recognize the inverted CG and TA base pairs. Therefore, the recognition of the CG and TA inversion sites to form a stable triplex DNA has been a long-standing goal for the triplex-forming technology. We now describe the design and synthesis of pseudo-deoxycytidine (ΨdC) derivatives for selective recognition of the CG base pair to expand the triplex-forming sequence. The aminopyridine-bearing ΨdC derivatives showed high selectivity and affinity toward the CG base pair in all neighboring base contexts. Remarkably, 3-methyl-2-aminopyridinyl−ΨdC (MeAP−ΨdC) formed a stable triplex with the promoter sequence of the hTERT gene containing four CG inversion sites, and effectively inhibited its transcription in human cancer cells. Thus, MeAP−ΨdC is expected to serve as a new starting point of triplex-forming oligonucleotides for a wide variety of genome-targeting applications.