Cobalt-Catalyzed sp2-C−H Activation: Intermolecular Heterocyclization with Allenes at Room Temperature
Corresponding Author
Dr. Neetipalli Thrimurtulu
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, 400076 India
Search for more papers by this authorArnab Dey
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, 400076 India
Search for more papers by this authorCorresponding Author
Prof. Debabrata Maiti
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, 400076 India
Search for more papers by this authorCorresponding Author
Prof. Chandra M. R. Volla
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, 400076 India
Search for more papers by this authorCorresponding Author
Dr. Neetipalli Thrimurtulu
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, 400076 India
Search for more papers by this authorArnab Dey
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, 400076 India
Search for more papers by this authorCorresponding Author
Prof. Debabrata Maiti
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, 400076 India
Search for more papers by this authorCorresponding Author
Prof. Chandra M. R. Volla
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, 400076 India
Search for more papers by this authorGraphical Abstract
Abstract
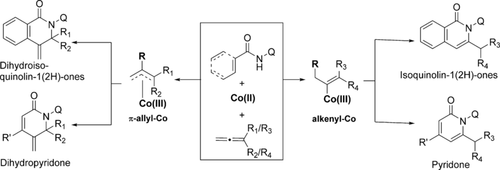
The reactivity of allenes in transition-metal-catalyzed C−H activation chemistry is governed by the formation of either alkenyl–metal (M–alkenyl) or metal–π-allyl intermediates. Although either protonation or a β-hydride elimination is feasible with a M–alkenyl intermediate, cyclization has remained unexplored to date. Furthermore, due to the increased steric hindrance, the regioselectivity for the intramolecular cyclization of the metal–π-allyl intermediate was hampered towards the more substituted side. To address these issues, a unified approach to synthesize a diverse array of biologically and pharmaceutically relevant heterocyclic moieties by cobalt-catalyzed directed C−H functionalization was envisioned. Upon successful implementation, the present strategy led to the regioselective formation of dihydroisoquinolin-1(2H)-ones, isoquinolin-1(2H)-ones, dihydropyridones, and pyridones.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201604956-sup-0001-misc_information.pdf3.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aU. Anwar, R. Grigg, M. Rasparini, V. Savic, V. Sridharan, Chem. Commun. 2000, 645–646;
- 1bH.-M. Chang, C.-H. Cheng, Org. Lett. 2000, 2, 3439–3442;
- 1cR. Zimmer, C. U. Dinesh, E. Nandanan, F. A. Khan, Chem. Rev. 2000, 100, 3067–3126;
- 1dA. S. K. Hashmi, Angew. Chem. Int. Ed. 2000, 39, 3590–3593;
10.1002/1521-3773(20001016)39:20<3590::AID-ANIE3590>3.0.CO;2-L CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 3737–3740;
- 1eS. Ma, in Handbook of Organopalladium Chemistry for Organic Synthesis, Wiley, New York, 2002, pp. 1491–1521;
10.1002/0471212466.ch62 Google Scholar
- 1fA. Hoffmann-Röder, N. Krause, Angew. Chem. Int. Ed. 2002, 41, 2933–2935;
10.1002/1521-3773(20020816)41:16<2933::AID-ANIE2933>3.0.CO;2-6 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 3057–3059;
- 1gJ. Löfstedt, K. Närhi, I. Dorange, J.-E. Bäckvall, J. Org. Chem. 2003, 68, 7243–7248;
- 1hS. Ma, Chem. Rev. 2005, 105, 2829–2872;
- 1iS. Ma, Pure Appl. Chem. 2006, 78, 197–208;
- 1j Modern Allene Chemistry (Eds.: ), Wiley-VCH, Weinheim, 2008, pp. 2–50;
- 1kM. Jeganmohan, C.-H. Cheng, Chem. Commun. 2008, 3101–3117;
- 1lO. Daugulis, H.-Q. Do, D. Shabashov, Acc. Chem. Res. 2009, 42, 1074–1086;
- 1mT. Bai, S. Ma, G. Jia, Coord. Chem. Rev. 2009, 253, 423–448;
- 1nX. Hong, M. C. Stevens, P. Liu, P. A. Wender, K. N. Houk, J. Am. Chem. Soc. 2014, 136, 17273–17283;
- 1oJ. Liu, Q. Liu, R. Franke, R. Jackstell, M. Beller, J. Am. Chem. Soc. 2015, 137, 8556–8563.
- 2
- 2aB. Ye, N. Cramer, J. Am. Chem. Soc. 2013, 135, 636–639;
- 2bD. N. Tran, N. Cramer, Angew. Chem. Int. Ed. 2013, 52, 10630–10634; Angew. Chem. 2013, 125, 10824–10828;
- 2cR. Zeng, J. Ye, C. Fu, S. Ma, Adv. Synth. Catal. 2013, 355, 1963–1970;
- 2dT.-J. Gong, W. Su, Z.-J. Liu, W.-M. Cheng, B. Xiao, Y. Fu, Org. Lett. 2014, 16, 330–333.
- 3Y. J. Zhang, E. Skucas, M. J. Krische, Org. Lett. 2009, 11, 4248–4250.
- 4
- 4aR. Zeng, C. Fu, S. Ma, J. Am. Chem. Soc. 2012, 134, 9597–9600;
- 4bR. Zeng, S. Wu, C. Fu, S. Ma, J. Am. Chem. Soc. 2013, 135, 18284–18287.
- 5
- 5aX.-F. Xia, Y.-Q. Wang, L.-L. Zhang, X.-R. Song, X.-Y. Liu, Y.-M. Liang, Chem. Eur. J. 2014, 20, 5087–5091;
- 5bA. Rodríguez, J. Albert, X. Ariza, J. Garcia, J. Granell, J. Farràs, A. La Mela, E. Nicolás, J. Org. Chem. 2014, 79, 9578–9585.
- 6
- 6aH. Wang, F. Glorius, Angew. Chem. Int. Ed. 2012, 51, 7318–7322; Angew. Chem. 2012, 124, 7430–7434;
- 6bH. Wang, B. Beiring, D.-G. Yu, K. D. Collins, F. Glorius, Angew. Chem. Int. Ed. 2013, 52, 12430–12434; Angew. Chem. 2013, 125, 12657–12661.
- 7D. N. Tran, N. Cramer, Angew. Chem. Int. Ed. 2010, 49, 8181–8184; Angew. Chem. 2010, 122, 8357–8360.
- 8Y. Kuninobu, P. Yu, K. Takai, Org. Lett. 2010, 12, 4274–4276.
- 9
- 9aQ. Chen, L. Ilies, E. Nakamura, J. Am. Chem. Soc. 2011, 133, 428–429;
- 9bL. Ilies, Q. Chen, X. Zeng, E. Nakamura, J. Am. Chem. Soc. 2011, 133, 5221–5223.
- 10
- 10aT. Yoshino, H. Ikemoto, S. Matsunaga, M. Kanai, Angew. Chem. Int. Ed. 2013, 52, 2207–2211; Angew. Chem. 2013, 125, 2263–2267;
- 10bT. Yoshino, H. Ikemoto, S. Matsunaga, M. Kanai, Chem. Eur. J. 2013, 19, 9142–9146;
- 10cH. Ikemoto, T. Yoshino, K. Sakata, S. Matsunaga, M. Kanai, J. Am. Chem. Soc. 2014, 136, 5424–5431.
- 11
- 11aK. Gao, N. Yoshikai, Acc. Chem. Res. 2014, 47, 1208–1219;
- 11bK. Gao, T. Yamakawa, N. Yoshikai, Synthesis 2014, 46, 2024–2039;
- 11cN. Yoshikai, ChemCatChem 2015, 7, 732–734;
- 11dJ. Wu, N. Yoshikai, Angew. Chem. Int. Ed. 2016, 55, 336–340; Angew. Chem. 2016, 128, 344–348.
- 12
- 12aH. Wang, J. Koeller, W. Liu, L. Ackermann, Chem. Eur. J. 2015, 21, 15525–15528;
- 12bJ. Li, L. Ackermann, Angew. Chem. Int. Ed. 2015, 54, 8551–8554; Angew. Chem. 2015, 127, 8671–8674;
- 12cR. Mei, H. Wang, S. Warratz, S. A. Macgregor, L. Ackermann, Chemistry 2016, 1–6;
- 12dM. Moselage, J. Li, L. Ackermann, ACS Catal. 2016, 6, 498–525.
- 13
- 13aL. Grigorjeva, O. Daugulis, Org. Lett. 2014, 16, 4688–4690;
- 13bL. Grigorjeva, O. Daugulis, Org. Lett. 2015, 17, 1204–1207;
- 13cT. T. Nguyen, L. Grigorjeva, O. Daugulis, ACS Catal. 2016, 6, 551–554.
- 14
- 14aD. Kalsi, B. Sundararaju, Org. Lett. 2015, 17, 6118–6121;
- 14bJ. R. Hummel, J. A. Ellman, J. Am. Chem. Soc. 2015, 137, 490–498;
- 14cM. Sen, B. Emayavaramban, N. Barsu, J. R. Premkumar, B. Sundararaju, ACS Catal. 2016, 6, 2792–2796.
- 15
- 15aR. C. Larock, J. M. Zenner, J. Org. Chem. 1995, 60, 482–483;
- 15bR. C. Larock, Y. He, W. W. Leong, X. Han, M. D. Refvik, J. M. Zenner, J. Org. Chem. 1998, 63, 2154–2160.
- 16
- 16aV. G. Zaitsev, D. Shabashov, O. Daugulis, J. Am. Chem. Soc. 2005, 127, 13154–13155;
- 16bF.-R. Gou, X.-C. Wang, P.-F. Huo, H.-P. Bi, Z.-H. Guan, Y.-M. Liang, Org. Lett. 2009, 11, 5726–5729;
- 16cD. Shabashov, O. Daugulis, J. Am. Chem. Soc. 2010, 132, 3965–3972;
- 16dY. Ano, M. Tobisu, N. Chatani, J. Am. Chem. Soc. 2011, 133, 12984–12986;
- 16eH. Shiota, Y. Ano, Y. Aihara, Y. Fukumoto, N. Chatani, J. Am. Chem. Soc. 2011, 133, 14952–14955;
- 16fW. R. Gutekunst, R. Gianatassio, P. S. Baran, Angew. Chem. Int. Ed. 2012, 51, 7507–7510; Angew. Chem. 2012, 124, 7625–7628;
- 16gY. Ano, M. Tobisu, N. Chatani, Org. Lett. 2012, 14, 354–357;
- 16hS. Asako, L. Ilies, E. Nakamura, J. Am. Chem. Soc. 2013, 135, 17755–17757;
- 16iY. Aihara, N. Chatani, J. Am. Chem. Soc. 2013, 135, 5308–5311;
- 16jG. Rouquet, N. Chatani, Angew. Chem. Int. Ed. 2013, 52, 11726–11743; Angew. Chem. 2013, 125, 11942–11959;
- 16kY. Aihara, N. Chatani, Chem. Sci. 2013, 4, 664–670;
- 16lG. Rouquet, N. Chatani, Chem. Sci. 2013, 4, 2201–2208;
- 16mL. Grigorjeva, O. Daugulis, Org. Lett. 2014, 16, 4684–4687;
- 16nT. Matsubara, S. Asako, L. Ilies, E. Nakamura, J. Am. Chem. Soc. 2014, 136, 646–649;
- 16oL. Grigorjeva, O. Daugulis, Angew. Chem. Int. Ed. 2014, 53, 10209–10212; Angew. Chem. 2014, 126, 10373–10376;
- 16pR. K. Rit, M. R. Yadav, K. Ghosh, A. K. Sahoo, Tetrahedron 2015, 71, 4450–4459;
- 16qX. Yang, G. Shan, L. Wang, Y. Rao, Tetrahedron Lett. 2016, 57, 819–836.
- 17
- 17aW. S. Wadsworth, W. D. Emmons, J. Am. Chem. Soc. 1961, 83, 1733–1738;
- 17bG. Hutton, T. Jolliff, H. Mitchell, S. Warren, Tetrahedron Lett. 1995, 36, 7905–7908.
- 18CCDC 1463918 (2 a), 1465049 (2 d), 1473152 (2 k), 1465183 (2 q), 1465051 (3 j), 1465053 (3 l), 1465184 (3 o), 1465054 (4), and 1473589 (intermediate A) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.