Isolation and Asymmetric Total Synthesis of Perforanoid A
Chao Lv
School of Pharmaceutical Sciences, Shandong University, Jinan, Shandong, 250012 PR China
These authors contributed equally to this work.
Search for more papers by this authorDr. Xiaohui Yan
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650204 PR China
These authors contributed equally to this work.
Search for more papers by this authorQian Tu
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Shenzhen, 518055 China
Search for more papers by this authorDr. Yingtong Di
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650204 PR China
Search for more papers by this authorDr. Chunmao Yuan
The Key Laboratory of Chemistry for Natural Products of Guizhou Province and Chinese Academy of Sciences, 550002 China
Search for more papers by this authorDr. Xin Fang
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650204 PR China
Search for more papers by this authorProf. Dr. Yaacove Ben-David
The Key Laboratory of Chemistry for Natural Products of Guizhou Province and Chinese Academy of Sciences, 550002 China
Search for more papers by this authorLei Xia
The Key Laboratory of Chemistry for Natural Products of Guizhou Province and Chinese Academy of Sciences, 550002 China
Search for more papers by this authorProf. Dr. Jianxian Gong
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yuemao Shen
School of Pharmaceutical Sciences, Shandong University, Jinan, Shandong, 250012 PR China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhen Yang
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaojiang Hao
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650204 PR China
Search for more papers by this authorChao Lv
School of Pharmaceutical Sciences, Shandong University, Jinan, Shandong, 250012 PR China
These authors contributed equally to this work.
Search for more papers by this authorDr. Xiaohui Yan
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650204 PR China
These authors contributed equally to this work.
Search for more papers by this authorQian Tu
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Shenzhen, 518055 China
Search for more papers by this authorDr. Yingtong Di
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650204 PR China
Search for more papers by this authorDr. Chunmao Yuan
The Key Laboratory of Chemistry for Natural Products of Guizhou Province and Chinese Academy of Sciences, 550002 China
Search for more papers by this authorDr. Xin Fang
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650204 PR China
Search for more papers by this authorProf. Dr. Yaacove Ben-David
The Key Laboratory of Chemistry for Natural Products of Guizhou Province and Chinese Academy of Sciences, 550002 China
Search for more papers by this authorLei Xia
The Key Laboratory of Chemistry for Natural Products of Guizhou Province and Chinese Academy of Sciences, 550002 China
Search for more papers by this authorProf. Dr. Jianxian Gong
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yuemao Shen
School of Pharmaceutical Sciences, Shandong University, Jinan, Shandong, 250012 PR China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhen Yang
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Shenzhen, 518055 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaojiang Hao
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650204 PR China
Search for more papers by this authorGraphical Abstract
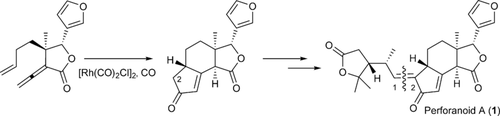
When life gives you limonoids: A novel limonoid, perforanoid A, was isolated, and an asymmetric total synthesis was achieved in 10 steps. The key steps are chiral tertiary aminonaphthol mediated enantioselective alkenylation of an aldehyde to an allylic alcohol, Pd-catalyzed coupling of the allylic alcohol with vinyl ether to form the γ-lactone ring, and cyclopentenone ring formation through a Rh-catalyzed Pauson–Khand reaction.
Abstract
A novel limonoid, perforanoid A, was isolated, and an asymmetric total synthesis was achieved in 10 steps. The key steps are chiral tertiary aminonaphthol mediated enantioselective alkenylation of an aldehyde to an allylic alcohol, Pd-catalyzed coupling of the allylic alcohol with vinyl ether to form the γ-lactone ring, and cyclopentenone ring formation through a Rh-catalyzed Pauson–Khand reaction. Preliminary studies show that perforanoid A is cytotoxic towards HEL, K562, and CB3 tumor cell lines.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201602783-sup-0001-misc_information.pdf6.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A recent review of triterpenoid isolation and structure determination:
- 1aJ. D. Connolly, R. A. Hill, Nat. Prod. Rep. 2010, 27, 79;
- 1bQ.-G. Tan, X.-D. Luo, Chem. Rev. 2011, 111, 7437;
- 1cX. Fang, Y.-T. Di, X.-J. Hao, Curr. Org. Chem. 2011, 15, 1363.
- 2
- 2aK.-H. Qui, C. Angele, R. Claude, N.-N. Hanh, N.-V. Kinh, K.-H. Francoise, J. Nat. Prod. 2001, 64, 634;
- 2bX. Fang, Y.-T. Di, H.-P. He, H.-Y. Liu, Z. Zhang, Y.-L. Ren, Z.-L. Gao, S. Gao, X.-J. Hao, Org. Lett. 2008, 10, 1905;
- 2cX. Fang, Q. Zhang, C.-J. Tan, S.-Z. Mu, Y. Lv, Y.-B. Lu, Q.-T. Zheng, Y.-T. Di, X.-J. Hao, Tetrahedron 2009, 65, 7408;
- 2dX.-H. Yan, Y.-T. Di, X. Fang, S.-Y. Yan, H.-P. He, S.-L. Li, Y. Lv, X.-J. Hao, Phytochemistry 2011, 72, 508;
- 2eC.-M. Yuan, Y. Zhang, G.-H. Tang, Y.-T. Di, M.-M. Cao, X.-Y. Wang, G.-Y. Zuo, S.-L. Li, H.-M. Hua, H.-P. He, X.-J. Hao, J. Nat. Prod. 2012, 76, 327.
- 3K.-K. Chiruvella, V. Kari, B. Choudhary, M. Nambiar, R.-G. Ghanta, S.-C. Raghavan, FEBS Lett. 2008, 582, 4066.
- 4
- 4aG. E. Brandt, M. D. Schmidt, T. E. Prisinzano, B. S. Blagg, J. Med. Chem. 2008, 51, 6495;
- 4bJ. Cui, Z. Deng, M. Xu, P. Proksch, Q. Li, W. Lin, Helv. Chim. Acta 2009, 92, 139;
- 4cR.-Y. Kuo, K. Qian, S. L. Morris-Natschke, K. H. Lee, Nat. Prod. Rep. 2009, 26, 1321.
- 5A.-K.-M.-S. Rahman, A.-K.-A. Chowdhury, H.-A. Ali, S.-Z. Raihan, M.-S. Ali, L. Nahar, S.-D. Sarker, J. Nat. Med. 2009, 63, 41.
- 6S.-E. Lee, M.-R. Kim, J.-H. Kim, G.-R. Takeoka, T.-W. Kim, B.-S. Park, Phytomedicine 2008, 15, 533.
- 7
- 7aY. Fukuyama, T. Tokoroyama, T. Kubota, Tetrahedron Lett. 1972, 13, 3401;
10.1016/S0040-4039(01)94055-9 Google Scholar
- 7bH. Okamura, K. Yamauchi, K. Miyawaki, T. Iwagawa, M. Nakatani, Tetrahedron Lett. 1997, 38, 263;
- 7cT. Tokoroyama, Y. Fukuyama, T. Kubota, K. Yokotani, J. Chem. Soc. Perkin Trans. 1 1981, 1557;
- 7dS. Trudeau, J. P. Morken, Org. Lett. 2005, 7, 5465.
- 8
- 8aG.-E. Veitch, E. Beckmann, B.-J. Burke, A. Boyer, S.-L. Maslen, S.-V. Ley, Angew. Chem. Int. Ed. 2007, 46, 7629; Angew. Chem. 2007, 119, 7773;
- 8bG.-E. Veitch, E. Beckmann, B.-J. Burke, A. Boyer, C. Ayats, S.-V. Ley, Angew. Chem. Int. Ed. 2007, 46, 7633; Angew. Chem. 2007, 119, 7777;
- 8cJ.-M. Faber, C.-M. Williams, Chem. Commun. 2011, 47, 2258;
- 8dJ.-M. Faber, W.-A. Eger, C.-M. Williams, J. Org. Chem. 2012, 77, 8913;
- 8eS. Yamashita, A. Naruko, Y. Nakazawa, L. Zhao, Y. Hayashi, M. Hirama, Angew. Chem. Int. Ed. 2015, 54, 8538; Angew. Chem. 2015, 127, 8658.
- 9Recently selected references for natural product synthesis by Pauson–Khand reactions:
- 9aQ. Xiao, W.-W. Ren, Z.-X. Chen, T.-W. Sun, Y. Li, Q.-D. Ye, J.-X. Gong, F.-K. Meng, L. You, Y.-F. Liu, M.-Z. Zhao, L.-M. Xu, Z.-H. Shan, Y.-F. Tang, J.-H. Chen, Z. Yang, Angew. Chem. Int. Ed. 2011, 50, 7373; Angew. Chem. 2011, 123, 7511;
- 9bY. Yang, X. Fu, J. Chen, H. Zhai, Angew. Chem. Int. Ed. 2012, 51, 9825; Angew. Chem. 2012, 124, 9963;
- 9cQ. Liu, G.-Z. Yue, N. Wu, G. Lin, Y. Z. Li, J.-M. Quan, C.-C. Li, G.-X. Wang, Z. Yang, Angew. Chem. Int. Ed. 2012, 51, 12072; Angew. Chem. 2012, 124, 12238;
- 9dJ. Huang, L.-C. Fang, R. Long, L.-L. Shi, H.-J. Shen, C.-C. Li, Z. Yang, Org. Lett. 2013, 15, 4018;
- 9eL. Jørgensen, S. J. McKerrall, C. A. Kuttruff, F. Ungeheuer, J. Felding, P. S. Baran, Science 2013, 341, 878;
- 9fL. You, X.-T. Liang, L.-M. Xu, Y.-F. Wang, J.-J. Zhang, Q. Su, Y.-H. Li, B. Zhang, S.-L. Yang, J.-H. Chen, Z. Yang, J. Am. Chem. Soc. 2015, 137, 10120.
- 10
- 10aF. Inagaki, C. Mukai, Org. Lett. 2006, 8, 1217;
- 10bY. Hayashi, N. Miyakoshi, S. Kitagaki, C. Mukai, Org. Lett. 2008, 10, 2385;
- 10cT. Hirose, N. Miyakoshi, C. Mukai, J. Org. Chem. 2008, 73, 1061.
- 11
- 11aM. A. Evans, J. P. Morken, Org. Lett. 2005, 7, 3367;
- 11bM. A. Evans, J. P. Morken, Org. Lett. 2005, 7, 3371.
- 12
- 12aS. A. M. W. van den Broek, P. G. W. Rensen, F. L. van Delft, F. P. J. T. Rutjes, Eur. J. Org. Chem. 2010, 5906;
- 12bS. W. Haynes, P. K. Sydor, C. Corre, L. Song, G. L. Challis, J. Am. Chem. Soc. 2011, 133, 1793.
- 13V. Sofiyev, G. Navarro, D. Trauner, Org. Lett. 2008, 10, 149.
- 14J.-X. Ji, L.-Q. Qiu, C. W. Yip, A. S. C. Chan, J. Org. Chem. 2003, 68, 1589.
- 15Y. Zhao, V. Snieckus, Org. Lett. 2014, 16, 390.
- 16sp3–sp2 Suzuki reaction:
- 16aS. R. Chemler, D. Trauner, S. J. Danishefsky, Angew. Chem. Int. Ed. 2001, 40, 4544;
10.1002/1521-3773(20011217)40:24<4544::AID-ANIE4544>3.0.CO;2-N CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 4676;
- 16bM. Ohba, N. Kawase, T. Fujii, J. Am. Chem. Soc. 1996, 118, 8250;
- 16cD. F. Meng, S. J. Danishefsky, Angew. Chem. Int. Ed. 1999, 38, 1485;
10.1002/(SICI)1521-3773(19990517)38:10<1485::AID-ANIE1485>3.0.CO;2-1 CAS PubMed Web of Science® Google ScholarAngew. Chem. 1999, 111, 1582;10.1002/(SICI)1521-3757(19990517)111:10<1582::AID-ANGE1582>3.0.CO;2-S Web of Science® Google Scholar
- 16dD. Trauner, J. B. Schwarz, S. J. Danishefsky, Angew. Chem. Int. Ed. 1999, 38, 3542;
10.1002/(SICI)1521-3773(19991203)38:23<3542::AID-ANIE3542>3.0.CO;2-I CAS PubMed Web of Science® Google ScholarAngew. Chem. 1999, 111, 3756;10.1002/(SICI)1521-3757(19991203)111:23<3756::AID-ANGE3756>3.0.CO;2-9 Web of Science® Google Scholar
- 16eC. R. Johnson, M. W. Miller, A. Golebiowski, H. Sundram, M. B. Ksebati, Tetrahedron Lett. 1994, 35, 8991.
- 17Negeshi reaction:
- 17aT. Hatakeyama, N. Nakagawa, M. Nakamura, Org. Lett. 2009, 11, 4496;
- 17bJ. Zhou, G. C. Fu, J. Am. Chem. Soc. 2003, 125, 12527;
- 17cS. L. Wiskur, A. Korte, G. C. Fu, J. Am. Chem. Soc. 2004, 126, 82.
- 18Kumada reaction:
- 18aA. Guérinot, S. Reymond, J. Cossy, Angew. Chem. Int. Ed. 2007, 46, 6521; Angew. Chem. 2007, 119, 6641;
- 18bH. Ohmiya, H. Yorimitsu, K. Oshima, Org. Lett. 2006, 8, 3093;
- 18cG. Cahiez, C. Duplais, A. Moyeux, Org. Lett. 2007, 9, 3253.
- 19G. A. Molander, O. A. Argintaru, Org. Lett. 2014, 16, 1904.
- 20D. Fernández González, J. P. Brand, J. Waser, Chem. Eur. J. 2010, 16, 9457.
- 21T. Ok, A. Jeon, J. Lee, J. H. Lim, C. S. Hong, H.-S. Lee, J. Org. Chem. 2007, 72, 7390.
- 22CCDC 1445838 (7) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 23
- 23aU. Ramulu, D. Ramesh, S. Rajaram, S. P. Reddy, K. Venkatesham, Y. Venkateswarlu, Tetrahedron: Asymmetry 2012, 23, 117;
- 23bK. Kuramochi, S. Nagata, H. Itaya, Y. Matsubara, T. Sunoki, H. Uchiro, K.-i. Takao, S. Kobayashi, Tetrahedron 2003, 59, 9743.
- 24S. Álvarez, H. Khanwalkar, R. Álvarez, C. Erb, C. Martínez, F. Rodríguez-Barrios, P. Germain, H. Gronemeyer, A. R. de Lera, ChemMedChem 2009, 4, 1630.
- 25J. M. Seco, E. Quiñoá, R. Riguera, Chem. Rev. 2004, 104, 17.
- 26Z. Li, L. Jia, J. Wang, X. Wu, H. Hao, Y. Wu, H. Xu, Z. Wang, G. Shi, C. Lu, Y. Shen, Eur. J. Med. Chem. 2014, 87, 346.