Copper-Catalyzed Insertion into Heteroatom–Hydrogen Bonds with Trifluorodiazoalkanes
Stephen Hyde
University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA UK
Search for more papers by this authorDr. Janis Veliks
University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA UK
Search for more papers by this authorDr. Benoît Liégault
University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA UK
Institut Charles Gerhardt Montpellier, UMR-CNRS 5253, AM2N, ENSCM, 8 rue de l'Ecole Normale, 34296 Montpellier Cedex 5, France
Search for more papers by this authorDr. David Grassi
University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA UK
Search for more papers by this authorDr. Marc Taillefer
Institut Charles Gerhardt Montpellier, UMR-CNRS 5253, AM2N, ENSCM, 8 rue de l'Ecole Normale, 34296 Montpellier Cedex 5, France
Search for more papers by this authorCorresponding Author
Prof. Véronique Gouverneur
University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA UK
Search for more papers by this authorStephen Hyde
University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA UK
Search for more papers by this authorDr. Janis Veliks
University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA UK
Search for more papers by this authorDr. Benoît Liégault
University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA UK
Institut Charles Gerhardt Montpellier, UMR-CNRS 5253, AM2N, ENSCM, 8 rue de l'Ecole Normale, 34296 Montpellier Cedex 5, France
Search for more papers by this authorDr. David Grassi
University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA UK
Search for more papers by this authorDr. Marc Taillefer
Institut Charles Gerhardt Montpellier, UMR-CNRS 5253, AM2N, ENSCM, 8 rue de l'Ecole Normale, 34296 Montpellier Cedex 5, France
Search for more papers by this authorCorresponding Author
Prof. Véronique Gouverneur
University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA UK
Search for more papers by this authorGraphical Abstract
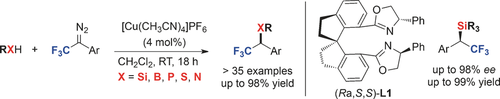
Suits all sorts: An efficient copper-catalyzed carbenoid insertion of 2,2,2-trifluorodiazoethane and 1-aryl 2,2,2-trifluorodiazoethanes into Si−H, B−H, P−H, S−H, and N−H bonds produced CF3-containing products in high yields (see scheme). Catalytic asymmetric Si−H and B−H bond insertion reactions were also developed with chiral bisoxazoline ligands, such as (Ra,S,S)-L1.
Abstract
Copper-catalyzed Si−H, B−H, P−H, S−H, and N−H insertion reactions of 2,2,2-trifluoro-1-diazoethane and 1-aryl 2,2,2-trifluorodiazoethanes generated a large number of new fluorine-containing chemical entities for medicinal chemists. With selected Si−H and B−H insertion reactions, we demonstrate successful extension to asymmetric catalysis.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201511954-sup-0001-misc_information.pdf37.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aT. Furuya, A. S. Kamlet, T. Ritter, Nature 2011, 473, 470;
- 1bJ.-A. Ma, D. Cahard, Chem. Rev. 2008, 108, PR 1;
- 1cO. A. Tomashenko, V. V. Grushin, Chem. Rev. 2011, 111, 4475;
- 1dD. L. Browne, Angew. Chem. Int. Ed. 2014, 53, 1482; Angew. Chem. 2014, 126, 1506;
- 1eC. Alonso, E. M. de Marigorta, G. Rubiales, F. Palacios, Chem. Rev. 2015, 115, 1847;
- 1fJ. Charpentier, N. Frîh, A. Togni, Chem. Rev. 2015, 115, 650;
- 1gX. Liu, C. Xu, M. Wang, Q. Liu, Chem. Rev. 2015, 115, 683;
- 1hX.-H. Xu, K. Matsuzaki, N. Shibata, Chem. Rev. 2015, 115, 731;
- 1iC. Ni, M. Hu, J. Hu, Chem. Rev. 2015, 115, 765;
- 1jS. Preshlock, M. Tredwell, V. Gouverneur, Chem. Rev. 2015, DOI: 10.1021/acs.chemrev.5b00493;
- 1kS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320.
- 2
- 2aY. Zhao, J. Hu, Angew. Chem. Int. Ed. 2012, 51, 1033; Angew. Chem. 2012, 124, 1057;
- 2bA. T. Brusoe, J. F. Hartwig, J. Am. Chem. Soc. 2015, 137, 8460.
- 3
- 3aW. Song, S. Lackner, L. Ackermann, Angew. Chem. Int. Ed. 2014, 53, 2477; Angew. Chem. 2014, 126, 2510;
- 3bG. L. Tolnai, A. Székely, Z. Makó, T. Gáti, J. Daru, T. Bihari, A. Stirling, Z. Novák, Chem. Commun. 2015, 51, 4488.
- 4T. Umemoto, Y. Gotoh, J. Fluorine Chem. 1986, 31, 213.
- 5H. Mimura, K. Kawada, T. Yamashita, T. Sakamoto, Y. Kikugawa, J. Fluorine Chem. 2010, 131, 477.
- 6
- 6aM. P. Doyle, T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley, New York, 1998;
- 6bH. M. L. Davies, S. J. Hedley, Chem. Soc. Rev. 2007, 36, 1109;
- 6cD. Gillingham, N. Fei, Chem. Soc. Rev. 2013, 42, 4918;
- 6dA. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire, M. A. McKervey, Chem. Rev. 2015, 115, 9981.
- 7
- 7aB. Morandi, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 938; Angew. Chem. 2010, 122, 950;
- 7bB. Morandi, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 4294; Angew. Chem. 2010, 122, 4390.
- 8
- 8aJ. R. Denton, D. Sukumaran, H. M. L. Davies, Org. Lett. 2007, 9, 2625;
- 8bE. Emer, J. Twilton, M. Tredwell, S. Calderwood, T. L. Collier, B. Liégault, M. Taillefer, V. Gouverneur, Org. Lett. 2014, 16, 6004.
- 9H. Luo, G. Wu, Y. Zhang, J. Wang, Angew. Chem. Int. Ed. 2015, 54, 14503; Angew. Chem. 2015, 127, 14711.
- 10For selected copper-catalyzed carbenoid insertion reactions, see: S−H insertion:
- 10aP. Yates, J. Am. Chem. Soc. 1952, 74, 5376;
- 10bH. Brunner, K. Wutz, M. P. Doyle, Monatsh. Chem. 1990, 121, 755;
- 10cY. Z. Zhang, S. F. Zhu, Y. Cai, H. X. Mao, Q. L. Zhou, Chem. Commun. 2009, 5362;
- 10dS. F. Zhu, Q. L. Zhou, Acc. Chem. Res. 2012, 45, 1365;
- 10eV. Tyagi, R. B. Bonn, R. Fasan, Chem. Sci. 2015, 6, 2488; O−H insertion:
- 10fT. C. Maier, G. C. Fu, J. Am. Chem. Soc. 2006, 128, 4594;
- 10gC. Chen, S.-F. Zhu, B. Liu, L.-X. Wang, Q.-L. Zhou, J. Am. Chem. Soc. 2007, 129, 12616;
- 10hY. Liang, H. Zhou, Z.-X. Yu, J. Am. Chem. Soc. 2009, 131, 17783.
- 11For selected rhodium-catalyzed insertion reactions, see: S−H insertion:
- 11aR. Paulissen, E. Hayez, A. J. Hubert, P. Teyssie, Tetrahedron Lett. 1974, 15, 607;
10.1016/S0040-4039(01)82283-8 Google Scholar
- 11bX. Zhang, M. Ma, J. Wang, ARKIVOC 2003, ii, 84;
- 11cH. E. Bartrum, D. C. Blakemore, C. J. Moody, C. J. Hayes, Tetrahedron 2013, 69, 2276;
- 11dB. Xu, S. F. Zhu, Z. C. Zhang, Z. X. Yu, Y. Ma, Q. L. Zhou, Chem. Sci. 2014, 5, 1442; N−H insertion:
- 11eB. Xu, S. F. Zhu, X. L. Xie, J. J. Shen, Q. L. Zhou, Angew. Chem. Int. Ed. 2011, 50, 11483; Angew. Chem. 2011, 123, 11685; Si−H insertion:
- 11fV. Bagheri, M. P. Doyle, J. Taunton, E. E. Claxton, J. Org. Chem. 1988, 53, 6158.
- 12For a review, see Ref. [10d]; for selected asymmetric X−H insertion reactions with α-diazoesters, see:
- 12aR. T. Buck, M. P. Doyle, M. J. Drysdale, L. Ferris, D. C. Forbes, D. Haigh, C. J. Moody, N. D. Pearson, Q.-L. Zhou, Tetrahedron Lett. 1996, 37, 7631;
- 12bRef. [10f];
- 12cS. F. Zhu, B. Xu, G. P. Wang, Q. L. Zhou, J. Am. Chem. Soc. 2012, 134, 436;
- 12dQ. Q. Cheng, S. F. Zhu, Y. Z. Zhang, X. L. Xie, Q. L. Zhou, J. Am. Chem. Soc. 2013, 135, 14094.
- 13J. A. Hirsch, Top. Stereochem. 1967, 3, 199.
10.1002/9780470147108.ch4 Google Scholar
- 14C. Hansch, A. Leo, R. W. Taft, Chem. Rev. 1991, 91, 165.
- 15
- 15aB. Greedy, J. M. Paris, T. Vidal, V. Gouverneur, Angew. Chem. Int. Ed. 2003, 42, 3291; Angew. Chem. 2003, 115, 3413;
- 15bY. H. Lam, C. Bobbio, I. R. Cooper, V. Gouverneur, Angew. Chem. Int. Ed. 2007, 46, 5106; Angew. Chem. 2007, 119, 5198;
- 15cS. C. Wilkinson, O. Lozano, M. Schuler, M. C. Pacheco, R. Salmon, V. Gouverneur, Angew. Chem. Int. Ed. 2009, 48, 7083; Angew. Chem. 2009, 121, 7217.
- 16M. C. Pirrung, H. Liu, A. T. Morehead, J. Am. Chem. Soc. 2002, 124, 1014.
- 17B. Liu, S. F. Zhu, W. Zhang, C. Chen, Q. L. Zhou, J. Am. Chem. Soc. 2007, 129, 5834.
- 18The copper-catalyzed asymmetric Si−H insertion of methyl α-diazophenylacetate with dimethylphenylsilane was reported by Zhou and co-workers. (Sa,S,S)-L1 and (Ra,S,S)-L1 led to 21 and 81 % ee, respectively: Y. Z. Zhang, S. F. Zhu, L. X. Wang, Q. L. Zhou, Angew. Chem. Int. Ed. 2008, 47, 8496; Angew. Chem. 2008, 120, 8624.
- 19I. Fleming, R. Henning, D. C. Parker, H. E. Plaut, P. E. J. Sanderson, J. Chem. Soc. Perkin Trans. 1 1995, 317.
- 20T. Fujisawa, Y. Onogawa, A. Sato, T. Mitsuya, M. Shimizu, Tetrahedron 1998, 54, 4267.