Chiral Cyclopentadienyl Iridium(III) Complexes Promote Enantioselective Cycloisomerizations Giving Fused Cyclopropanes
Dr. Michael Dieckmann
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Search for more papers by this authorYun-Suk Jang
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Search for more papers by this authorCorresponding Author
Prof. Dr. Nicolai Cramer
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsaSearch for more papers by this authorDr. Michael Dieckmann
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Search for more papers by this authorYun-Suk Jang
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Search for more papers by this authorCorresponding Author
Prof. Dr. Nicolai Cramer
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, 1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsaSearch for more papers by this authorGraphical Abstract
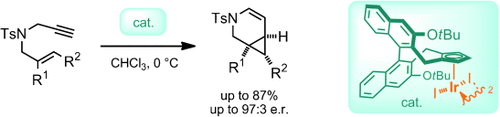
Be selective! A set of chiral CpxIrIII complexes (Cpx=chiral cyclopentadienyl) based on atropchiral cyclopentadienyl ligands are presented. The complexes, in particular the tert-butoxy-substituted derivative (see picture), are shown to promote the asymmetric cycloisomerization of enynes to form fused cyclopropanes with high enantioselectivities.
Abstract
The cyclopentadienyl (Cp) group is a very important ligand for many transition-metal complexes which have been applied in catalysis. The availability of chiral cyclopentadienyl ligands (Cpx) lags behind other ligand classes, thus hampering the investigation of enantioselective processes. We report a library of chiral CpxIrIII complexes equipped with an atropchiral Cp scaffold. A robust complexation procedure reliably provides CpxIrIII complexes with tunable counterions. In a proof-of-concept application, the iodide-bearing members are shown to be highly selective for enyne cycloisomerization reactions. The dehydropiperidine-fused cyclopropane products are formed in good yields and enantioselectivities.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201506483_sm_miscellaneous_information.pdf3.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Hartwig, Organotransition Metal Chemistry: From Bonding to Catalysis, University Science Books, Sausalito, 2010.
- 2
- 2aR. L. Halterman, K. P. C. Vollhardt, M. E. A. Welker, J. Am. Chem. Soc. 1987, 109, 8105–8107;
- 2bS. L. Colletti, R. L. Halterman, Organometallics 1991, 10, 3438–3448;
- 2cZ. Chen, R. L. Halterman, Synlett 1990, 103–104;
- 2dS. L. Colletti, R. L. Halterman, Tetrahedron Lett. 1989, 30, 3513–3516;
- 2eZ. Li, A. Vasella, Helv. Chim. Acta 1996, 79, 2201–2218;
- 2fR. L. Halterman, L. D. Crow, Tetrahedron Lett. 2003, 44, 2907–2909.
- 3
- 3aR. L. Haltermann, Chem. Rev. 1992, 92, 965–994;
- 3bR. L. Halterman, K. P. C. Vollhardt, Organometallics 1988, 7, 883–892;
- 3cR. L. Halterman, K. P. C. Vollhardt, Tetrahedron Lett. 1986, 27, 1461–1464;
- 3dC. Krüger, R. Goddard, M. Nolte, J. Organomet. Chem. 1993, 459, 107–115;
- 3eA. Gutnov, B. Heller, H.-J. Drexler, A. Spannenberg, G. Oehme, Organometallics 2003, 22, 1550–1553;
- 3fH. Schumann, O. Stenzel, S. Dechert, F. Girgsdies, J. Blum, D. Gelman, R. L. Halterman, Eur. J. Inorg. Chem. 2002, 211–219;
- 3gA. Gutnov, H.-J. Drexler, A. Spannenberg, G. Oehme, B. Heller, Organometallics 2004, 23, 1002–1009;
- 3hG. P. McGlacken, C. T. O′Brien, A. C. Whitwood, I. J. S. Fairlamb, Organometallics 2007, 26, 3722–3728;
- 3iZ. R. Turner, J.-C. Buffet, D. O′Hare, Organometallics 2014, 33, 3891–3903;
- 3jR. Laï, J.-C. Daran, D. Nuel, M. Sanz, N. Summerton, N. Vanthuyne, A. Zaragori-Benedetti, Dalton Trans. 2013, 42, 7980–7990;
- 3kB. Heller, A. Gutnov, C. Fischer, H.-J. Drexler, A. Spannenberg, D. Redkin, C. Sundermann, B. Sundermann, Chem. Eur. J. 2007, 13, 1117–1128;
- 3lA. Gutnov, B. Heller, C. Fischer, H.-J. Drexler, A. Spannenberg, Angew. Chem. Int. Ed. 2004, 43, 3795–3797; Angew. Chem. 2004, 116, 3883–3886.
- 4
- 4aB. Ye, N. Cramer, Science 2012, 338, 504–506;
- 4bB. Ye, N. Cramer, J. Am. Chem. Soc. 2013, 135, 636–639.
- 5
- 5aB. Ye, N. Cramer, Acc. Chem. Res. 2015, 48, 1308–1318;
- 5bB. Ye, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 7896–7899; Angew. Chem. 2014, 126, 8030–8033;
- 5cM. D. Wodrich, B. Ye, J. F. Gonthier, C. Corminboeuf, N. Cramer, Chem. Eur. J. 2014, 20, 15409–15418;
- 5dB. Ye, P. A. Donets, N. Cramer, Angew. Chem. Int. Ed. 2014, 53, 507–511; Angew. Chem. 2014, 126, 517–521;
- 5eJ. Zheng, S.-L. You, Angew. Chem. Int. Ed. 2014, 53, 13244–13247; Angew. Chem. 2014, 126, 13460–13463;
- 5fJ. Zheng, S.-B. Wang, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2015, 137, 4880–4883;
- 5gB. Ye, N. Cramer, Synlett 2015, 26, 1490–1495;
- 5hG. Y. Song, W. N. O. Wiley, Z. M. Hou, J. Am. Chem. Soc. 2014, 136, 12209–12212.
- 6D. Bellus, S. V. Ley, R. Noyori, M. Regitz, P. J. Reider, E. Schaumann, I. Shinkai, E. J. Thomas, B. M. Trost, in Science of Synthesis—Methods of Molecular Transformations, Vol. 1, (Ed. ), Wiley, New York, 2001.
- 7C. White, A. Yates, P. M. Maitlis, Inorg. Synth. 1992, 29, 228–234.
- 8D. M. Heinekey, J. M. Millar, T. K. Koetzle, N. G. Payne, K. W. Zilm, J. Am. Chem. Soc. 1990, 112, 909–919.
- 9CCDC 1409033 (2 d) and 1410893 (5 g) contain supplementary crystallographic information. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 10E. Benedetti, A. Simonneau, A. Hours, H. Amouri, A. Penoni, G. Palmisano, M. Malacria, J.-P. Goddard, L. Fensterbank, Adv. Synth. Catal. 2011, 353, 1908–1912.
- 11A. Fürstner, P. W. Davies, Angew. Chem. Int. Ed. 2007, 46, 3410–3449; Angew. Chem. 2007, 119, 3478–3519.
- 12For reviews, see:
- 12aI. D. G. Watson, F. D. Toste, Chem. Sci. 2012, 3, 2899–2919;
- 12bL. Zhang, J. Sun, S. A. Kozmin, Adv. Synth. Catal. 2006, 348, 2271–2296;
- 12cR. Dorel, A. M. Echavarren, J. Org. Chem. 2015, DOI: 10.1021/acs.joc.5b01106.
- 13For examples with AuI and PtII, see:
- 13aA. Fürstner, F. Stelzer, H. Szillat, J. Am. Chem. Soc. 2001, 123, 11863–11869;
- 13bF. Schröder, C. Tugny, E. Salanouve, H. Clavier, L. Giordano, D. Moraleda, Y. Gimbert, V. Mouriès-Mansuy, J.-P. Goddard, L. Fensterbank, Organometallics 2014, 33, 4051–4056;
- 13cD.-H. Zhang, Y. Wei, M. Shi, Chem. Eur. J. 2012, 18, 7026–7029;
- 13dJ. B. Xia, W.-B. Liu, T.-M. Wang, S.-L. You, Chem. Eur. J. 2010, 16, 6442–6446;
- 13eS. Anjum, J. Marco-Contelles, Tetrahedron 2005, 61, 4793–4803;
- 13fJ. Blum, H. Beer-Kraft, Y. Badrieh, J. Org. Chem. 1995, 60, 5567–5569;
- 13gK. Fourmy, S. Mallet-Ladeira, O. Dechy-Cabaret, M. Gouygou, Dalton Trans. 2014, 43, 6728–6734;
- 13hE. Soriano, P. Ballesteros, J. Marco-Contelles, J. Org. Chem. 2004, 69, 8018–8023;
- 13iN. M. Groome, E. E. Elboray, M. W. Inman, H. A. Dondas, R. M. Phillips, C. Kilner, R. Grigg, Chem. Eur. J. 2013, 19, 2180–2184.
- 14For example with further metals, see:
- 14aS. Son, S. Y. Kim, ChemistryOpen 2012, 1, 169–172;
- 14bK. Ota, S. I. Lee, J.-M. Tang, M. Takachi, H. Nakai, T. Morimoto, H. Sakurai, K. Kataoka, N. Chatani, J. Am. Chem. Soc. 2009, 131, 15203–15211;
- 14cS. Y. Kim, J. Org. Chem. 2010, 75, 1281–1284;
- 14dN. Chatani, H. Inoue, T. Morimoto, T. Muto, S. Murai, J. Org. Chem. 2001, 66, 4433–4436;
- 14eS. M. Stevenson, E. T. Newcomb, E. M. Ferreira, Chem. Commun. 2014, 50, 5239–5241;
- 14fS. H. Sim, J. H. Park, Y. K. Chung, Adv. Synth. Catal. 2010, 352, 317–322;
- 14gH. M. Oh, J. E. Park, J. Kim, J. H. Kim, Y. K. Kang, Y. K. Chung, Chem. Eur. J. 2014, 20, 9024–9036;
- 14hJ. Böhmer, R. Grigg, J. D. Marchbank, Chem. Commun. 2002, 768–769.
- 15
- 15aT. Shibata, Y. Kobayashi, S. Maekawa, N. Toshida, K. Takagi, Tetrahedron 2005, 61, 9018–9024;
- 15bM. Barbazanges, M. Augé, J. Moussa, H. Amouri, C. Aubert, C. Desmartes, L. Fensterbank, V. Gandon, M. Malacria, C. Ollivier, Chem. Eur. J. 2011, 17, 13789–13794.
- 16
- 16aA. Pradal, C.-M. Chao, P. Y. Toullec, V. Michelet, Beilstein J. Org. Chem. 2011, 7, 1021–1029;
- 16bK. Yavari, P. Aillard, Y. Zhang, F. Nutter, P. Retailleau, A. Voituriez, A. Marinetti, Angew. Chem. Int. Ed. 2014, 53, 861–865; Angew. Chem. 2014, 126, 880–884;
- 16cP. Pérez-Galán, E. Herrero-Gómez, D. T. Hog, N. J. A. Martin, F. Maseras, A. M. Echavarren, Chem. Sci. 2011, 2, 141–149;
- 16dH. Teller, M. Corbet, L. Mantilli, G. Gopakumar, R. Goddard, W. Thiel, A. Fürstner, J. Am. Chem. Soc. 2012, 134, 15331–15342;
- 16eW. Wang, J. Yang, F. Wang, M. Shi, Organometallics 2011, 30, 3859–3869.
- 17
- 17aD. Brissy, M. Skander, H. Jullien, P. Retailleau, A. Marinetti, Org. Lett. 2009, 11, 2137–2139;
- 17bD. Brissy, M. Skander, P. Retailleau, G. Frison, A. Marinetti, Organometallics 2009, 28, 140–151.
- 18
- 18aT. Nishimura, Y. Maeda, T. Hayashi, Org. Lett. 2011, 13, 3674–3677;
- 18bT. Nishimura, Y. Takiguchi, Y. Maeda, T. Hayashi, Adv. Synth. Catal. 2013, 355, 1374–1382;
- 18cK. Matsutomi, K. Noguchi, K. Tanaka, J. Am. Chem. Soc. 2014, 136, 7626–7630.
- 19Terminal alkyne substrates in Au catalysis typically furnish 5-exo-dig products (Refs. [16c], [16e]). A single example of 6-endo-dig cyclization gives 23 % yield and 22 % ee (Ref. [16a]).
- 20V. S. Sridevi, W. Y. Fan, W. K. Leong, Organometallics 2007, 26, 1173–1177.
- 21See the Supporting Information for details.