Blending Cr2O3 into a NiO–Ni Electrocatalyst for Sustained Water Splitting
Ming Gong
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Wu Zhou
Materials Science & Technology Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831 (USA)
These authors contributed equally to this work.
Search for more papers by this authorMichael James Kenney
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Yingpeng Wu
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
Search for more papers by this authorDr. Bingan Lu
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
School of Physics and Electronics, Hunan University, Changsha, 410082 (China)
Search for more papers by this authorDr. Meng-Chang Lin
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
Green Energy and Environment Research Laboratories, Industrial Technology Research Insititute, Hsinchu, 31040 (Taiwan, ROC)
Search for more papers by this authorDr. Di-Yan Wang
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
Department of Chemistry, National Taiwan Normal University, Taipei, 11677 (Taiwan, ROC)
Search for more papers by this authorDr. Jiang Yang
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
Search for more papers by this authorProf. Bing-Joe Hwang
Department of Chemical Engineering, National Taiwan University of Science and Technology, Taipei, 106 (Taiwan, ROC)
Search for more papers by this authorCorresponding Author
Prof. Hongjie Dai
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)Search for more papers by this authorMing Gong
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Wu Zhou
Materials Science & Technology Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831 (USA)
These authors contributed equally to this work.
Search for more papers by this authorMichael James Kenney
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Yingpeng Wu
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
Search for more papers by this authorDr. Bingan Lu
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
School of Physics and Electronics, Hunan University, Changsha, 410082 (China)
Search for more papers by this authorDr. Meng-Chang Lin
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
Green Energy and Environment Research Laboratories, Industrial Technology Research Insititute, Hsinchu, 31040 (Taiwan, ROC)
Search for more papers by this authorDr. Di-Yan Wang
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
Department of Chemistry, National Taiwan Normal University, Taipei, 11677 (Taiwan, ROC)
Search for more papers by this authorDr. Jiang Yang
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
Search for more papers by this authorProf. Bing-Joe Hwang
Department of Chemical Engineering, National Taiwan University of Science and Technology, Taipei, 106 (Taiwan, ROC)
Search for more papers by this authorCorresponding Author
Prof. Hongjie Dai
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)
Department of Chemistry, Stanford University, Stanford, CA 94305 (USA)Search for more papers by this authorGraphical Abstract
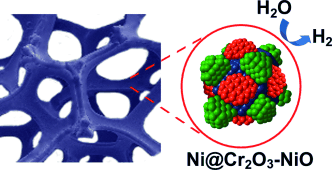
A triphase electrocatalyst composed of a Cr2O3-blended NiO coating on Ni nanocores (CrNN catalyst) synthesized on metal-foam substrates showed superior activity and stability for the hydrogen-evolution reaction in basic solutions. Using the CrNN catalyst, sustained electrolysis of water was achieved at a voltage lower than 1.5 V for at least 500 hours.
Abstract
The rising H2 economy demands active and durable electrocatalysts based on low-cost, earth-abundant materials for water electrolysis/photolysis. Here we report nanoscale Ni metal cores over-coated by a Cr2O3-blended NiO layer synthesized on metallic foam substrates. The Ni@NiO/Cr2O3 triphase material exhibits superior activity and stability similar to Pt for the hydrogen-evolution reaction in basic solutions. The chemically stable Cr2O3 is crucial for preventing oxidation of the Ni core, maintaining abundant NiO/Ni interfaces as catalytically active sites in the heterostructure and thus imparting high stability to the hydrogen-evolution catalyst. The highly active and stable electrocatalyst enables an alkaline electrolyzer operating at 20 mA cm−2 at a voltage lower than 1.5 V, lasting longer than 3 weeks without decay. The non-precious metal catalysts afford a high efficiency of about 15 % for light-driven water splitting using GaAs solar cells.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201504815_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. W. Crabtree, M. S. Dresselhaus, M. V. Buchanan, Phys. Today 2004, 57, 39–44;
- 1bM. S. Dresselhaus, I. L. Thomas, Nature 2001, 414, 332–337;
- 1cD. G. Nocera, Acc. Chem. Res. 2012, 45, 767–776;
- 1dM. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori, N. S. Lewis, Chem. Rev. 2010, 110, 6446–6473.
- 2P. Häussinger, R. Lohmüller, A. M. Watson, Ullmann′s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000.
10.1002/14356007.a13_297 Google Scholar
- 3
- 3aM. Carmo, D. L. Fritz, J. Merge, D. Stolten, Int. J. Hydrogen Energy 2013, 38, 4901–4934;
- 3bC. L. Choi, J. Feng, Y. Li, J. Wu, A. Zak, R. Tenne, H. Dai, Nano Res. 2013, 6, 921–928; Y. Zhang, J. Shi, G. Han, M. Li, Q. Ji, D. Ma, Y. Zhang, C. Li, X. Lang, Y. Zhang, Nano Res. 2015, 8, 1–10;
- 3cJ. Feng, M. Gong, M. Kenney, J. Wu, B. Zhang, Y. Li, H. Dai, Nano Res. 2015, 8, 1–7;
- 3dM. Gong, W. Zhou, M.-C. Tsai, J. Zhou, M. Guan, M.-C. Lin, B. Zhang, Y. Hu, D.-Y. Wang, J. Yang, S. J. Pennycook, B.-J. Hwang, H. Dai, Nat. Commun. 2014, 5, 0;
- 3eM. J. Kenney, M. Gong, Y. Li, J. Z. Wu, J. Feng, M. Lanza, H. Dai, Science 2013, 342, 836–840;
- 3fK. Zeng, D. Zhang, Prog. Energy Combust. Sci. 2010, 36, 307–326.
- 4Y. Liang, Y. Li, H. Wang, H. Dai, J. Am. Chem. Soc. 2013, 135, 2013–2036.
- 5
- 5aD. Friebel, M. W. Louie, M. Bajdich, K. E. Sanwald, Y. Cai, A. M. Wise, M.-J. Cheng, D. Sokaras, T.-C. Weng, R. Alonso-Mori, R. C. Davis, J. R. Bargar, J. K. Nørskov, A. Nilsson, A. T. Bell, J. Am. Chem. Soc. 2015, 137, 1305–1313;
- 5bM. Gong, H. Dai, Nano Res. 2015, 8, 23–39;
- 5cM. Gong, Y. Li, H. Wang, Y. Liang, J. Z. Wu, J. Zhou, J. Wang, T. Regier, F. Wei, H. Dai, J. Am. Chem. Soc. 2013, 135, 8452–8455;
- 5dM. W. Kanan, D. G. Nocera, Science 2008, 321, 1072–1075;
- 5eE. J. Popczun, J. R. McKone, C. G. Read, A. J. Biacchi, A. M. Wiltrout, N. S. Lewis, R. E. Schaak, J. Am. Chem. Soc. 2013, 135, 9267–9270;
- 5fJ. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough, Y. Shao-Horn, Science 2011, 334, 1383–1385;
- 5gD.-Y. Wang, M. Gong, H.-L. Chou, C.-J. Pan, H.-A. Chen, Y. Wu, M.-C. Lin, M. Guan, J. Yang, C.-W. Chen, Y.-L. Wang, B.-J. Hwang, C.-C. Chen, H. Dai, J. Am. Chem. Soc. 2015, 137, 1587–1592.
- 6
- 6aY. Sun, M. Delucchi, J. Ogden, Int. J. Hydrogen Energy 2011, 36, 11116–11127;
- 6bJ. R. McKone, S. C. Marinescu, B. S. Brunschwig, J. R. Winkler, H. B. Gray, Chem. Sci. 2014, 5, 865–878.
- 7
- 7aI. Olefjord, Mater. Sci. Eng. 1980, 42, 161–171;
- 7bM. F. Montemor, M. G. S. Ferreira, N. E. Hakiki, M. D. Belo, Corros. Sci. 2000, 42, 1635–1650;
- 7cP. Stefanov, D. Stoychev, M. Stoycheva, T. Marinova, Mater. Chem. Phys. 2000, 65, 212–215.
- 8B. Beverskog, I. Puigdomenech, Corros. Sci. 1997, 39, 43–57.
- 9
- 9aC. R. Cox, J. Z. Lee, D. G. Nocera, T. Buonassisi, Proc. Natl. Acad. Sci. USA 2014, 111, 14057–14061;
- 9bJ. Luo, J.-H. Im, M. T. Mayer, M. Schreier, M. K. Nazeeruddin, N.-G. Park, S. D. Tilley, H. J. Fan, M. Graetzel, Science 2014, 345, 1593–1596.
- 10
- 10aM. A. Green, K. Emery, Y. Hishikawa, W. Warta, E. D. Dunlop, Prog. Photovoltaics 2015, 23, 1–9;
- 10bO. Khaselev, J. A. Turner, Science 1998, 280, 425–427.