Carbamate-Catalyzed Enantioselective Bromolactamization
Dr. Yi An Cheng
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
Search for more papers by this authorWesley Zongrong Yu
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ying-Yeung Yeung
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
Department of Chemistry, The Chinese University of Hong Kong, Shatin, Hong Kong (China)
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)Search for more papers by this authorDr. Yi An Cheng
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
Search for more papers by this authorWesley Zongrong Yu
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ying-Yeung Yeung
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)
Department of Chemistry, The Chinese University of Hong Kong, Shatin, Hong Kong (China)
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543 (Singapore)Search for more papers by this authorGraphical Abstract
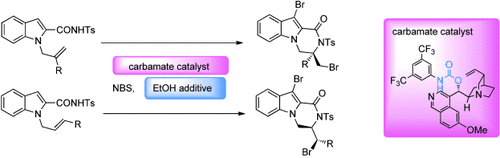
A splash of EtOH: A highly facile, efficient, and enantioselective bromolactamization of olefinic amides was effected by a carbamate catalyst and ethanol additive. The amide substrates undergo N-cyclization predominantly to give a diverse range of enantioenriched bromolactam products which contain up to two chiral centers. Ts=4-toluenesulfonyl.
Abstract
A highly facile, efficient, and enantioselective bromolactamization of olefinic amides was effected by a carbamate catalyst and ethanol additive. The amide substrates underwent N-cyclization predominantly to give a diverse range of enantioenriched bromolactam products containing up to two stereogenic centers.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201504724_sm_miscellaneous_information.pdf5.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on halocyclization, see:
- 1aA. Castellanos, S. P. Fletcher, Chem. Eur. J. 2011, 17, 5766;
- 1bC. K. Tan, L. Zhou, Y.-Y. Yeung, Synlett 2011, 1335;
- 1cU. Hennecke, Chem. Asian J. 2012, 7, 456;
- 1dS. E. Denmark, W. E. Kuester, M. T. Burk, Angew. Chem. Int. Ed. 2012, 51, 10938; Angew. Chem. 2012, 124, 11098;
- 1eS. R. Chemler, M. T. Bovino, ACS Catal. 2013, 3, 1076;
- 1fK. Murai, H. Fujioka, Heterocycles 2013, 87, 763;
- 1gC. K. Tan, Y.-Y. Yeung, Chem. Commun. 2013, 49, 7985;
- 1hC. K. Tan, W. Z. Yu, Y.-Y. Yeung, Chirality 2014, 26, 328;
- 1iY. A. Cheng, W. Z. Yu, Y.-Y. Yeung, Org. Biomol. Chem. 2014, 12, 2333.
- 2For selected recent efforts on the development of catalytic enantioselective halolactonization, see:
- 2aK. Murai, J. Nakajima, A. Nakamura, N. Hyogo, H. Fujioka, Chem. Asian J. 2014, 9, 3511;
- 2bK. Murai, N. Shimizu, H. Fujioka, Chem. Commun. 2014, 50, 12530;
- 2cH. Nakatsuji, Y. Sawamura, A. Sakakura, K. Ishihara, Angew. Chem. Int. Ed. 2014, 53, 6974; Angew. Chem. 2014, 126, 7094;
- 2dM. Wilking, C. G. Daniliuc, U. Hennecke, Synlett 2014, 1701;
- 2eT. Arai, N. Sugiyama, H. Masu, S. Kado, S. Yabe, M. Yamanaka, Chem. Commun. 2014, 50, 8287;
- 2fX. Han, C. Dong, H.-B. Zhou, Adv. Synth. Catal. 2014, 356, 1275.
- 3For selected recent efforts on the development of catalytic enantioselective haloaminocyclization, see:
- 3aC. B. Tripathi, S. Murkherjee, Org. Lett. 2014, 16, 3368;
- 3bY. Cai, P. Zhou, X. Liu, J. Zhao, L. Lin, X. Feng, Chem. Eur. J. 2015, 21, 6386;
- 3cW. Xie, G. Jiang, H. Liu, J. Hu, X. Pan, H. Zhang, X. Wan, Y. Lai, D. Ma, Angew. Chem. Int. Ed. 2013, 52, 12924; Angew. Chem. 2013, 125, 13162.
- 4For selected recent efforts on the development of catalytic enantioselective haloetherification, see:
- 4aC. H. Müller, C. Rösner, U. Hennecke, Chem. Asian J. 2014, 9, 2162;
- 4bD. W. Tay, G. Y. C. Leung, Y.-Y. Yeung, Angew. Chem. Int. Ed. 2014, 53, 5161; Angew. Chem. 2014, 126, 5261;
- 4cZ. Ke, C. K. Tan, F. Chen, Y.-Y. Yeung, J. Am. Chem. Soc. 2014, 136, 5627.
- 5For elegant reports of enantioselective bromo-N-cyclization of olefinic carbamate substrates using a metal catalysts, see:
- 5aD. Huang, X. Liu, L. Li, Y. Cai, W. Liu, Y. Shi, J. Am. Chem. Soc. 2013, 135, 8101;
- 5bH. Huang, H. Pan, Y. Cai, M. Liu, H. Tian, Y. Shi, Org. Biomol. Chem. 2015, 13, 3566.
- 6
- 6aG. Cardillo, M. Orena, Tetrahedron 1990, 46, 3321;
- 6bK. E. Harding, T. H. Tiner in Comprehensive Organic Synthesis, Vol. 4 (Ed.: ), Pergamon, New York, 1991, p. 363.
- 7
- 7aA. Jaganathan, A. Garzan, D. C. Whitehead, R. J. Staples, B. Borhan, Angew. Chem. Int. Ed. 2011, 50, 2593; Angew. Chem. 2011, 123, 2641;
- 7bA. Jaganathan, R. J. Staples, B. Borhan, J. Am. Chem. Soc. 2013, 135, 14806;
- 7cQ. Yin, S.-L. You, Org. Lett. 2014, 16, 2426; Q. Yin, S.-L. You, Org. Lett. 2013, 15, 4266;
- 7dY.-M. Wang, J. Wu, C. Hoong, V. Rauniyar, F. D. Toste, J. Am. Chem. Soc. 2012, 134, 12928;
- 7eV. Rauniyar, A. D. Lackner, G. L. Hamilton, F. D. Toste, Science 2011, 334, 1681;
- 7fY. Kawato, A. Kubota, H. Ono, H. Egami, Y. Hamashima, Org. Lett. 2015, 17, 1244.
- 8Organocatalysts such as thioureas have been widely utilized in various asymmetric reactions. In contrast, carbamates have not been used as catalysts in organic transformations. For references on thiourea organocatalysts, see:
- 8aM. S. Sigman, E. N. Jacobsen, J. Am. Chem. Soc. 1998, 120, 4901;
- 8bH. B. Jang, J. W. Lee, C. E. Song, Cinchona Alkaloids in Synthesis and Catalysis: Ligands, Immobilization and Organocatalysis (Ed.: ), Wiley-VCH, Weinheim, 2009, chap. 8;
- 8cM. Kotke, P. R. Schreiner in Hydrogen Bonding in Organic Synthesis (Ed.: ), Wiley-VCH, Weinheim, 2009, chap. 6;
- 8dY. Xi, X. Shi, Chem. Commun. 2013, 49, 8583.
- 9
- 9aZ. Xiong, D. A. Gao, D. A. Cogan, D. R. Goldberg, M.-H. Hao, N. Moss, E. Pack, C. Pargellis, D. Skow, T. Trieselmann, B. Werneburg, A. White, Bioorg. Med. Chem. Lett. 2008, 18, 1994;
- 9bH. G. F. Richter, C. Freichel, J. Huwyler, T. Nakagawa, M. Nettekoven, J.-M. Plancher, S. O. Roche, F. Schuler, S. Taylor, C. Ullmer, R. Wiegand, Bioorg. Med. Chem. Lett. 2010, 20, 5713;
- 9cR. R. Forseth, E. M. Fox, D. W. Chung, B. J. Howlett, N. P. Keller, F. C. Schroeder, J. Am. Chem. Soc. 2011, 133, 9678;
- 9dR. Sekonyela, J. M. Palmer, J.-W. Bok, S. Jain, E. Berthier, R. Forseth, F. Schroeder, N. P. Keller, Plos One 2013, 8, e 62591;
- 9eD. Goldberg, A. Abeywardane, C. Miller, T. Morwick, M. Netherton, R. Snow, J. Wang, J.-P. Wu, Z. Xiong, US Pat. Appl. US 2006/0276453A1, 2006;
- 9fM, Nettekoven, J.-M. Plancher, H. Richter, O. Roche, S. Taylor, PCT Int. Appl. WO 2007/065820A1, 2007.
- 10
- 10aSee Ref. [4b];
- 10bY. Zhao, X. Jiang, Y.-Y. Yeung, Angew. Chem. Int. Ed. 2013, 52, 8597; Angew. Chem. 2013, 125, 8759;
- 10cL. Zhou, D. W. Tay, J. Chen, G. Y. C. Leung, Y.-Y. Yeung, Chem. Commun. 2013, 49, 4412;
- 10dX. Jiang, C. K. Tan, L. Zhou, Y.-Y. Yeung, Angew. Chem. Int. Ed. 2012, 51, 7771; Angew. Chem. 2012, 124, 7891;
- 10eC. K. Tan, C. Le, Y.-Y. Yeung, Chem. Commun. 2012, 48, 5793;
- 10fL. Zhou, J. Chen, C. K. Tan, Y.-Y. Yeung, J. Am. Chem. Soc. 2011, 133, 9164;
- 10gC. K. Tan, L. Zhou, Y.-Y. Yeung, Org. Lett. 2011, 13, 2738;
- 10hL. Zhou, C. K. Tan, X. Jiang, F. Chen, Y.-Y. Yeung, J. Am. Chem. Soc. 2010, 132, 15474.
- 11The details appear in the Supporting Information.
- 12The ee value of the bromolactamization product was highly dependent on the size of the alcohol additive, and much lower conversion was observed when a sterically bulky alcohol was used. In addition, the use of Et2O in place of EtOH as the additive led to a significant reduction in ee value, thus suggesting that the hydroxy group of EtOH might play a crucial role in the reaction. Thus, we speculate that the ethanol might involve in the enantiodetermining step with the involvement of hydrogen bonding. For details, see the Supporting Information.
- 13CCDC 1063550 (2 h) and 1063551 (5 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 14J. D. Crowley, D. A. McMorran, Top. Heterocycl. Chem. 2012, 28, 31.
- 15S. M. Ahmad, D. C. Braddock, G. Cansell, S. A. Hermitage, J. M. Redmonda, A. J. P. White, Tetrahedron Lett. 2007, 48, 5948.
- 16Since carbamate can undergo tautomerization, we cannot rule out the possibility that 3 d-Br might exist as the form of O-Br species instead of N-Br species.
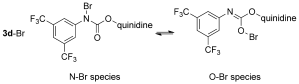
- 17Although the reaction rate between 3 d and NBS was slow, the reaction rate between 3 d and bromine source could be greatly enhanced by replacing NBS with TBCO.
- 18We also attempted to cyclize an olefinic amide with a benzene core instead of an indole. The corresponding bromolactam could be obtained in good yield and appreciable enantioselectivity. For details, see the Supporting Information.