Easily Accessible Auxiliary for Palladium-Catalyzed Intramolecular Amination of C(sp2)H and C(sp3)H Bonds at δ- and ε-Positions†
Chao Wang
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorChangpeng Chen
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorJingyu Zhang
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorJian Han
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorQian Wang
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorKun Guo
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorPei Liu
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorMingyu Guan
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorCorresponding Author
Dr. Yingming Yao
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)Search for more papers by this authorCorresponding Author
Prof. Dr. Yingsheng Zhao
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)Search for more papers by this authorChao Wang
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorChangpeng Chen
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorJingyu Zhang
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorJian Han
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorQian Wang
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorKun Guo
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorPei Liu
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorMingyu Guan
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Search for more papers by this authorCorresponding Author
Dr. Yingming Yao
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)Search for more papers by this authorCorresponding Author
Prof. Dr. Yingsheng Zhao
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)
Key Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123 (P.R. China)Search for more papers by this authorWe are grateful to Dr. Aiwen Lei (Wuhan University) for helpful suggestions and comments on this manuscript. We gratefully acknowledge financial support from the Natural Science Foundation of Jiangsu Province of China (L210903913), and a start-up fund (Q410901212) from Soochow University for support of this work. The PAPD and Qing Lan Project are also gratefully acknowledged.
Graphical Abstract
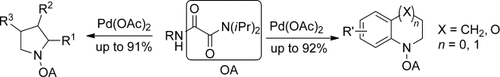
Remote access: The first application of an oxalyl amide to direct CH functionalizations at remote positions is reported. The results show both C(sp2)H and C(sp3)H bonds at δ- and ε-positions are effectively activated, thus giving tetrahydroquinolines, benzomorpholines, pyrrolidines, and indolines in moderate to excellent yields by palladium-catalyzed intramolecular CH amination.
Abstract
An easily synthesized and accessible N,O-bidentate auxiliary has been developed for selective CH activation under palladium catalysis. The novel auxiliary showed its first powerful application in CH functionalization of remote positions. Both C(sp2)H and C(sp3)H bonds at δ- and ε-positions were effectively activated, thus giving tetrahydroquinolines, benzomorpholines, pyrrolidines, and indolines in moderate to excellent yields by palladium-catalyzed intramolecular CH amination.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201404854_sm_miscellaneous_information.pdf8.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. Giri, B.-F. Shi, K. M. Engle, N. Maugel, J.-Q. Yu, Chem. Soc. Rev. 2009, 38, 3242;
- 1bO. Daugulis, H.-Q. Do, D. Shabashov, Acc. Chem. Res. 2009, 42, 1074;
- 1cT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 1dD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624;
- 1eH. M. L. Davies, J. Du Bois, J.-Q. Yu, Chem. Soc. Rev. 2011, 40, 1855;
- 1fB.-J. Li, Z.-J. Shi, Chem. Soc. Rev. 2012, 41, 5588;
- 1gK. M. Engle, T.-S. Mei, M. Wasa, J.-Q. Yu, Acc. Chem. Res. 2011, 44, 788;
- 1hP. B. Arockiam, C. Bruneau, P. H. Dixneuf, Chem. Rev. 2012, 112, 5879;
- 1iS. R. Neufeldt, M. S. Sanford, Acc. Chem. Res. 2012, 45, 936;
- 1jJ. J. Mousseau, A. B. Charette, Acc. Chem. Res. 2012, 45, 412;
- 1kJ. Bariwal, E. Van der Eycken, Chem. Soc. Rev. 2013, 42, 9283;
- 1lG. Rouquet, N. Chatani, Angew. Chem. 2013, 125, 11942;
10.1002/ange.201301451 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 11726;
- 1mM.-L. Louillat, F. W. Patureau, Chem. Soc. Rev. 2014, 43, 901.
- 2
- 2aB. D. Dangel, J. A. Johnson, D. Sames, J. Am. Chem. Soc. 2001, 123, 8149;
- 2bF.-R. Gou, X.-C. Wang, P.-F. Huo, H.-P. B. H.-P. Bi, Org. Lett. 2009, 11, 5726;
- 2cJ. J. Neumann, S. Rakshit, T. Dröge, F. Glorius, Angew. Chem. 2009, 121, 7024; Angew. Chem. Int. Ed. 2009, 48, 6892;
- 2dP. Thansandote, M. Lautens, Chem. Eur. J. 2009, 15, 5874;
- 2eM. Nakanishi, D. Katayev, C. Besnard, E. P. Kündig, Angew. Chem. 2011, 123, 7576; Angew. Chem. Int. Ed. 2011, 50, 7438;
- 2fG. He, Y. Zhao, S. Zhang, C. Lu, G. Chen, J. Am. Chem. Soc. 2012, 134, 3;
- 2gE. T. Nadres, O. Daugulis, J. Am. Chem. Soc. 2012, 134, 7;
- 2hS.-H. Cho, J. Yoon, S. Chang, J. Am. Chem. Soc. 2011, 133, 5996;
- 2iA. P. Antonchick, R. Samanta, K. Kulikov, J. Lategahn, Angew. Chem. 2011, 123, 8764;
10.1002/ange.201102984 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 8605.
- 3For examples of triflamide as a directing group, see:
- 3aJ.-J. Li, T.-S. Mei, J.-Q. Yu, Angew. Chem. 2008, 120, 6552; Angew. Chem. Int. Ed. 2008, 47, 6452;
- 3bT.-S. Mei, X. Wang, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 10806;
- 3cC. J. Vickers, T.-S. Mei, J.-Q. Yu, Org. Lett. 2010, 12, 2511;
- 3dK. S. L. Chan, M. Wasa, C. Ling, B. N. Laforteza, M. Miura, J.-Q. Yu, Nat. Chem. 2014, 6, 146.
- 4For examples of picolinic amide as a directing group, see:
- 4aV. G. Zaitsev, D. Shabashov, O. Daugulis, J. Am. Chem. Soc. 2005, 127, 13154;
- 4bD. Shabashov, O. Daugulis, J. Am. Chem. Soc. 2010, 132, 3965;
- 4cT. Truong, K. Klimovica, O. Daugulis, J. Am. Chem. Soc. 2013, 135, 9342;
- 4dY. Feng, Y. Wang, B. Landgraf, S. Liu, G. Chen, Org. Lett. 2010, 12, 3414;
- 4eY. Zhao, G. Chen, Org. Lett. 2011, 13, 4850;
- 4fG. He, C. Lu, Y. Zhao, W. A. Nack, G. Chen, Org. Lett. 2012, 14, 2944;
- 4gS.-Y. Zhang, Q. Li, G. He, W. A. Nack, G. Chen, J. Am. Chem. Soc. 2013, 135, 2124.
- 5X. Ye, Z. He, T. Ahmed, K. Weise, N. G. Akhmedov, J. L. Petersen, X. Shi, Chem. Sci. 2013, 4, 3712.
- 6
- 6aW. C. P. Tsang, N. Zheng, S. L. Buchwald, J. Am. Chem. Soc. 2005, 127, 14560;
- 6bJ. A. Jordan-Hore, C. Johansson, M. Gulias, E. M. Beck, M. J. Gaunt, J. Am. Chem. Soc. 2008, 130, 16184;
- 6cT.-S. Mei, L. Kou, S. Ma, K. M. Engle, J.-Q. Yu, Synthesis 2012, 44, 1778.
- 7
- 7aS. Rousseaux, B. Liegault, K. Fagnou, Chem. Sci. 2012, 3, 244;
- 7bT. Saget, N. Cramer, Angew. Chem. 2012, 124, 13014;
10.1002/ange.201207959 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 12842;
- 7cJ.-X. Yan, H. Li, X.-W. Liu, J.-L. Shi, X. Wang, Z.-J. Shi, Angew. Chem. 2014, 126, 5045; Angew. Chem. Int. Ed. 2014, 53, 4945.
- 8
- 8aA. R. Katritzky, S. Rachwal, B. Rachwal, Tetrahedron 1996, 52, 15031;
- 8bV. Sridharan, P. A. Suryavanshi, J. C. Menéndez, Chem. Rev. 2011, 111, 7157.
- 9M. Wasa, J.-Q. Yu, J. Am. Chem. Soc. 2008, 130, 14058.
- 10
- 10aR. Giri, J.-Q. Yu, J. Am. Chem. Soc. 2008, 130, 14082;
- 10bY. Lu, D.-H. Wang, K. M. Engle, J.-Q. Yu, J. Am. Chem. Soc. 2010, 132, 5916;
- 10cG. Li, D. Leow, L. Wan, J.-Q. Yu, Angew. Chem. 2012, 124, 1283; Angew. Chem. Int. Ed. 2012, 51, 1245.
- 11
- 11aL. C. Misal Castro, N. Chatani, Chem. Eur. J. 2014, 20, 4548–4553;
- 11bB. V. S. Reddy, G. Revathi, A. S. Reddy, J. S. Yadav, Tetrahedron Lett. 2011, 52, 5926.
- 12A. John, K. M. Nicholas, J. Org. Chem. 2012, 77, 5600.
- 13
- 13aD. A. Evans, T. C. Britton, J. A. Ellman, Tetrahedron Lett. 1987, 28, 6141;
- 13bY. Feng, G. Chen, Angew. Chem. 2010, 122, 970; Angew. Chem. Int. Ed. 2010, 49, 958.
- 14
- 14aV. Snieckus, Chem. Rev. 1990, 90, 879;
- 14bK. Muto, J. Yamaguchi, A. Lei, K. Itami, J. Am. Chem. Soc. 2013, 135, 16384.
- 15
- 15aA. P. Wells, W. Kitching, Organometallics 1992, 11, 2750;
- 15bR. Giri, X. Chen, J.-Q. Yu, Angew. Chem. 2005, 117, 2150;
10.1002/ange.200462884 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2112.
- 16T. Saget, N. Cramer, Angew. Chem. 2013, 125, 8019;
10.1002/ange.201303816 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 7865.
- 17CCDC 1013832 (8) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.