A Conical Intersection Controls the Deactivation of the Bacterial Luciferase Fluorophore†
Dr. Samer Gozem
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/
These authors contributed equally to this work.
Search for more papers by this authorDr. Ekaterina Mirzakulova
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/
These authors contributed equally to this work.
Search for more papers by this authorDr. Igor Schapiro
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/
Search for more papers by this authorDr. Federico Melaccio
Department of Biotechnology, Chemistry and Pharmacy, Università di Siena, via A. Moro 2, 53100 Siena (Italy)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ksenija D. Glusac
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/Search for more papers by this authorCorresponding Author
Prof. Dr. Massimo Olivucci
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/
Department of Biotechnology, Chemistry and Pharmacy, Università di Siena, via A. Moro 2, 53100 Siena (Italy)
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/Search for more papers by this authorDr. Samer Gozem
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/
These authors contributed equally to this work.
Search for more papers by this authorDr. Ekaterina Mirzakulova
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/
These authors contributed equally to this work.
Search for more papers by this authorDr. Igor Schapiro
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/
Search for more papers by this authorDr. Federico Melaccio
Department of Biotechnology, Chemistry and Pharmacy, Università di Siena, via A. Moro 2, 53100 Siena (Italy)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ksenija D. Glusac
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/Search for more papers by this authorCorresponding Author
Prof. Dr. Massimo Olivucci
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/
Department of Biotechnology, Chemistry and Pharmacy, Università di Siena, via A. Moro 2, 53100 Siena (Italy)
Chemistry Department, Bowling Green State University, Bowling Green, OH 43403 (USA) http://www.bgsu.lcpp.com/Search for more papers by this authorWe thank Dr. T. Domratcheva for helpful discussion. K.D.G. acknowledges support from the National Science Foundation (CHE-1055397 CAREER award). M.O. acknowledges support from the National Science Foundation (grant no. CHE-1152070), the Human Frontier Science Program Organization (grant RGP0049/2012CHE09-56776), and the EU-FP7 (Marie-Curie PIOF-GA-2012-332233). The authors are indebted to NSF-XSEDE and OSC for granted computer time. The European Cooperation in Science and Technology Action CM1002 is also acknowledged.
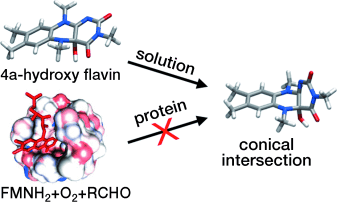
Graphical Abstract
How nature lights up flavins: 4a-hydroxy flavins display weak fluorescence and ultrafast excited-state decay in solution, but exhibit strong fluorescence when produced in a protein cavity. A joint experimental and theoretical study explains the fluorescence properties of these flavin adducts in terms of a deactivation pathway mediated by a conical intersection that becomes inaccessible in sterically constrained environments.
Abstract
The photophysics of flavins is highly dependent on their environment. For example, 4a-hydroxy flavins display weak fluorescence in solution, but exhibit strong fluorescence when bound to a protein. To understand this behavior, we performed temperature-dependent fluorescent studies on an N(5)-alkylated 4a-hydroxy flavin: the putative bacterial luciferase fluorophore. We find an increase in fluorescence quantum yield upon reaching the glass transition temperature of the solvent. We then employ multiconfigurational quantum chemical methods to map the excited-state deactivation path of the system. The result reveals a shallow but barrierless excited state deactivation path that leads to a conical intersection displaying an orthogonal out-of-plane distortion of the terminal pyrimidine ring. The intersection structure readily explains the observed spectroscopic behavior in terms of an excited-state barrier imposed by the rigid glass cavity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201404011_sm_miscellaneous_information.pdf687 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. Leenders, A. Van Hoek, M. Van Iersel, C. Veeger, A. J. Visser, Eur. J. Biochem. 1993, 218, 977–984.
- 2S. P. Laptenok, L. Bouzhir-Sima, J. C. Lambry, H. Myllykallio, U. Liebl, M. H. Vos, Proc. Natl. Acad. Sci. USA 2013, 110, 8924–8929.
- 3H. Yang, G. Luo, P. Karnchanaphanurach, T. M. Louie, I. Rech, S. Cova, L. Xun, X. S. Xie, Science 2003, 302, 262–266.
- 4D. Zhong, A. H. Zewail, Proc. Natl. Acad. Sci. USA 2001, 98, 11867–11872.
- 5J. Brazard, A. Usman, F. Lacombat, C. Ley, M. M. Martin, P. Plaza, L. Mony, M. Heijde, G. Zabulon, C. Bowler, J. Am. Chem. Soc. 2010, 132, 4935–4945.
- 6S. Ghisla, V. Massey, J. M. Lhoste, S. G. Mayhew, Biochemistry 1974, 13, 589–597.
- 7S. Ghisla, Methods Enzymol. 1980, 66, 360–373.
- 8C. T. Moonen, J. Vervoort, F. Mueller, Biochemistry 1984, 23, 4859–4867.
- 9C. T. Moonen, J. Vervoort, F. Müller, Biochemistry 1984, 23, 4868–4872.
- 10Y. T. Kao, C. Saxena, T. F. He, L. Guo, L. Wang, A. Sancar, D. Zhong, J. Am. Chem. Soc. 2008, 130, 13132–13139.
- 11D. Zhou, E. Mirzakulova, R. Khatmullin, I. Schapiro, M. Olivucci, K. D. Glusac, J. Phys. Chem. B 2011, 115, 7136–7143.
- 12G. Li, V. Sichula, K. D. Glusac, J. Phys. Chem. B 2008, 112, 10758–10764.
- 13J. Li, Z. Liu, C. Tan, X. Guo, L. Wang, A. Sancar, D. Zhong, Nature 2010, 466, 887–890.
- 14Y. T. Kao, C. Saxena, L. Wang, A. Sancar, D. Zhong, Proc. Natl. Acad. Sci. USA 2005, 102, 16128–16132.
- 15B. Lei, Q. Ding, S. C. Tu, Biochemistry 2004, 43, 15975–15982.
- 16S. D. Miller, S. H. Haddock, C. D. Elvidge, T. F. Lee, Proc. Natl. Acad. Sci. USA 2005, 102, 14181–14184.
- 17W. J. van Berkel, N. M. Kamerbeek, M. W. Fraaije, J. Biotechnol. 2006, 124, 670–689.
- 18J. W. Hastings, C. Balny, C. L. Peuch, P. Douzou, Proc. Natl. Acad. Sci. USA 1973, 70, 3468–3472.
- 19J. Vervoort, F. Muller, J. Lee, W. A. Van den Berg, C. T. Moonen, Biochemistry 1986, 25, 8062–8067.
- 20S. Ghisla, J. W. Hastings, V. Favaudon, J. M. Lhoste, Proc. Natl. Acad. Sci. USA 1978, 75, 5860–5863.
- 21J. W. Eckstein, K. W. Cho, P. Colepicolo, S. Ghisla, J. W. Hastings, T. Wilson, Proc. Natl. Acad. Sci. USA 1990, 87, 1466–1470.
- 22M. Kurfürst, S. Ghisla, J. W. Hastings, Proc. Natl. Acad. Sci. USA 1984, 81, 2990–2994.
- 23C. Hou, Y. J. Liu, N. Ferré, W. H. Fang, Chem. Eur. J. 2014, 20, 7979–7986.
- 24S. Ghisla, B. Entsch, V. Massey, M. Husein, Eur. J. Biochem. 1977, 76, 139–148.
- 25L. M. Wang, C. A. Angell, R. Richert, J. Chem. Phys. 2006, 125, 074505.
- 26J. Grilj, E. N. Laricheva, M. Olivucci, E. Vauthey, Angew. Chem. 2011, 123, 4589–4591;
10.1002/ange.201100015 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 4496–4498.
- 27D. F. Duxbury, Chem. Rev. 1993, 93, 381–433.
- 28A. Nakayama, T. Taketsugu, J. Phys. Chem. A 2011, 115, 8808–8815.
- 29K. L. Litvinenko, N. M. Webber, S. R. Meech, J. Phys. Chem. A 2003, 107, 2616–2623.
- 30K. Detmer, V. Massey, J. Biol. Chem. 1984, 259, 11265–11272.
- 31M. Merchán, R. González-Luque, T. Climent, L. Serrano-Andrés, E. Rodríguez, M. Reguero, D. Peláez, J. Phys. Chem. B 2006, 110, 26471–26476.
- 32R. González-Luque, M. Garavelli, F. Bernardi, M. Merchán, M. A. Robb, M. Olivucci, Proc. Natl. Acad. Sci. USA 2000, 97, 9379–9384.
- 33M. Drobizhev, T. E. Hughes, Y. Stepanenko, P. Wnuk, K. O’Donnell, J. N. Scott, P. R. Callis, A. Mikhaylov, L. Dokken, A. Rebane, Sci. Rep. 2012, 2, 688.
- 34J. Y. Hasegawa, S. Bureekaew, H. Nakatsuji, J. Photochem. Photobiol. A 2007, 189, 205–210.
- 35L. Salem, Acc. Chem. Res. 1979, 12, 87–92.
- 36P. B. Coto, A. Sinicropi, L. De Vico, N. Ferre, M. Olivucci, Mol. Phys. 2006, 104, 983–991.
- 37M. Barbatti, A. J. Aquino, J. J. Szymczak, D. Nachtigallová, P. Hobza, H. Lischka, Proc. Natl. Acad. Sci. USA 2010, 107, 21453–21458.
- 38D. Roca-Sanjuán, F. Aquilante, R. Lindh, WIREs Comput. Mol. Sci. 2012, 2, 585–603.
- 39I. Schapiro, M. N. Ryazantsev, W. J. Ding, M. M. Huntress, F. Melaccio, T. Andruniow, M. Olivucci, Aust. J. Chem. 2010, 63, 413–429.
- 40Z. T. Campbell, A. Weichsel, W. R. Montfort, T. O. Baldwin, Biochemistry 2009, 48, 6085–6094.
- 41N. A. Baker, D. Sept, S. Joseph, M. J. Holst, J. A. McCammon, Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041.
- 42M. H. M. Olsson, C. R. Søndergaard, M. Rostkowski, J. H. Jensen, J. Chem. Theory Comput. 2011, 7, 525–537.
- 43T. W. Cline, J. W. Hastings, J. Biol. Chem. 1974, 249, 4668–4669.
- 44T. Domratcheva, A. Udvarhelyi, A. R. Shahi, Methods Mol Biol (Ed.: ), Springer, New York, 2014, pp. 191–228.
- 45A. Udvarhelyi, T. Domratcheva, Photochem. Photobiol. 2011, 87, 554–563.
- 46K. Sadeghian, M. Bocola, M. Schütz, Phys. Chem. Chem. Phys. 2010, 12, 8840–8846.
- 47M. R. Silva-Junior, M. Mansurova, W. Gärtner, W. Thiel, ChemBioChem 2013, 14, 1648–1661.
- 48M. E. Martin, F. Negri, M. Olivucci, J. Am. Chem. Soc. 2004, 126, 5452–5464.
- 49A. Mukherjee, K. B. Weyant, J. Walker, C. M. Schroeder, J. Biol. Eng. 2012, 6, 20.
- 50A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. L. Belser, A. Von Zelewsky, Coord. Chem. Rev. 1988, 84, 85–277.
- 51B. O. Roos in Advances in Chemical Physics: Ab Initio Methods in Quantum Chemistry Part 2, Vol. 69 (Ed.: K. P. Lawley), Wiley, 1987, pp. 399–445.
- 52K. Andersson, P. A. Malmqvist, B. O. Roos, A. J. Sadlej, K. Wolinski, J. Phys. Chem. 1990, 94, 5483–5488.
- 53P. Z. El-Khoury, I. Schapiro, M. Huntress, F. Melaccio, S. Gozem, L. M. Frutos, M. Olivucci in CRC Handbook of Organic Photochemistry and Photobiology (Eds.: ), CRC, Taylor & Francis, Boca Raton, 2012, pp. 1029–1056.
- 54S. Gozem, F. Melaccio, H. L. Luk, S. Rinaldi, M. Olivucci, Chem. Soc. Rev. 2014, 43, 4019–4036.
- 55S. Gozem, M. Huntress, I. Schapiro, R. Lindh, A. A. Granovsky, C. Angeli, M. Olivucci, J. Chem. Theory Comput. 2012, 8, 4069–4080.
- 56F. Aquilante, L. De Vico, N. Ferré, G. Ghigo, P. A. Malmqvist, P. Neogrády, T. B. Pedersen, M. Pitonák, M. Reiher, B. O. Roos, L. Serrano-Andrés, M. Urban, V. Veryazov, R. Lindh, J. Comput. Chem. 2010, 31, 224–247.