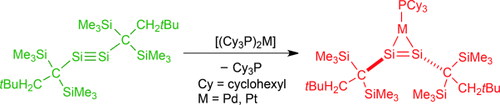Palladium and Platinum η2-Disilyne Complexes Bearing an Isolable Dialkyldisilyne as a Ligand†
Corresponding Author
Dr. Shintaro Ishida
Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578 (Japan)
Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578 (Japan)===Search for more papers by this authorRyutaro Sugawara
Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578 (Japan)
Search for more papers by this authorYoshifumi Misawa
Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Takeaki Iwamoto
Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578 (Japan)
Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578 (Japan)===Search for more papers by this authorCorresponding Author
Dr. Shintaro Ishida
Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578 (Japan)
Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578 (Japan)===Search for more papers by this authorRyutaro Sugawara
Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578 (Japan)
Search for more papers by this authorYoshifumi Misawa
Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Takeaki Iwamoto
Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578 (Japan)
Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578 (Japan)===Search for more papers by this authorThis work was supported by JSPS KAKENHI Grant Number 25248010 (T.I.), 25708004, and 25620020 (S.I.); MEXT KAKENHI Grant Number 24109004 (T.I.) (Grant-in-Aid for Scientific Research on Innovative Areas “Stimuli-responsive Chemical Species”). We thank Dr. Soji Shimizu and Prof. Nagao Kobayashi (Tohoku University) for measurement of UV/Vis-NIR spectroscopy.
Graphical Abstract
Not so alkyne like: A dialkyldisilyne (left, green) that can be isolated is synthesized and fully characterized. It coordinates to palladium and platinum in a η2-fashion giving complexes (red) with a trans-bent geometry, in contrast to η2-alkyne complexes. The complexes showed significant metallacycle character.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201308517_sm_miscellaneous_information.pdf3.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1N. Wiberg, S. K. Vasisht, G. Fischer, P. Mayer, Z. Anorg. Allg. Chem. 2004, 630, 1823–1828.
- 2A. Sekiguchi, R. Kinjo, M. Ichinohe, Science 2004, 305, 1755–1757.
- 3Y. Murata, M. Ichinohe, A. Sekiguchi, J. Am. Chem. Soc. 2010, 132, 16768–16770.
- 4
- 4aT. Sasamori, K. Hironaka, Y. Sugiyama, N. Takagi, S. Nagase, Y. Hosoi, Y. Furukawa, N. Tokitoh, J. Am. Chem. Soc. 2008, 130, 13856–13857;
- 4bT. Sasamori, J.-S. Han, K. Hironaka, N. Takagi, S. Nagase, N. Tokitoh, Pure Appl. Chem. 2010, 82, 603–612.
- 5Reviews for disilynes and related Group 14 elements multiple bonds, see:
- 5aP. P. Power, Chem. Rev. 1999, 99, 3463–3504;
- 5bM. Weidenbruch, J. Organomet. Chem. 2002, 646, 39–52;
- 5cP. P. Power, Chem. Commun. 2003, 2091–2101;
- 5dM. Weidenbruch, Angew. Chem. 2005, 117, 518–520;
10.1002/ange.200462273 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 514–516;
- 5eP. P. Power, Appl. Organomet. Chem. 2005, 19, 488–493;
- 5fP. P. Power, Organometallics 2007, 26, 4362–4372;
- 5gA. Sekiguchi, Pure Appl. Chem. 2008, 80, 447–457;
- 5hP. P. Power, Nature 2010, 463, 171–177;
- 5iR. C. Fischer, P. P. Power, Chem. Rev. 2010, 110, 3877–3927;
- 5jM. Asay, A. Sekiguchi, Bull. Chem. Soc. Jpn. 2012, 85, 1245–1261;
- 5kT. Sasamori, N. Tokitoh, Bull. Chem. Soc. Jpn. 2013, 86, 1005–1021.
- 6Base-stabilized bis(silylenes), valence isomers of disilynes, have been reported, see:
- 6aY. Wang, Y. Xie, P. Wei, R. B. King, H. F. Schaefer III, P. v. R. Schleyer, G. H. A. Robinson, Science 2008, 321, 1069–1071;
- 6bS. S. Sen, A. Jana, H. W. Roesky, C. Schulzke, Angew. Chem. 2009, 121, 8688–8690;
10.1002/ange.200902995 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 8536–8538;
- 6cD. Gau, R. Rodriguez, T. Kato, N. Saffon-Merceron, A. de Cozar, F. P. Cossío, A. Baceiredo, Angew. Chem. 2011, 123, 1124–1128; Angew. Chem. Int. Ed. 2011, 50, 1092–1096.
- 7Theoretical studies of disilynes, see:
- 7aM. Karni, Y. Apeloig, N. Takagi, S. Nagase, Organometallics 2005, 24, 6319–6330;
- 7bD. Auer, M. Kaupp, C. Strohmann, Organometallics 2005, 24, 6331–6337.
- 8A recent Review of the bonding character of transition-metal complexes; G. Frenking, N. Fröhlich, Chem. Rev. 2000, 100, 717–774.
- 9R. Stegmann, G. Frenking, Organometallics 1995, 14, 5308–5315.
- 10Y. Kuramoto, N. Sawai, Y. Fujiwara, M. Sumimoto, Y. Nakao, H. Sato, S. Sakaki, Organometallics 2005, 24, 3655–3663.
- 11T. Yamaguchi, A. Sekiguchi, M. Driess, J. Am. Chem. Soc. 2010, 132, 14061–14063.
- 12Regioselective carbolithiation of silylethylenes, see:
- 12aL. F. Cason, H. G. Brooks, J. Org. Chem. 1954, 19, 1278–1282;
- 12bJ. E. Mulvaney, Z. G. Gardlund, J. Org. Chem. 1965, 30, 917–920;
- 12cP. F. Hudrlik, D. Peterson, J. Am. Chem. Soc. 1975, 97, 1464–1468;
- 12dJ. Clayden in Organolithiums: Selectivity for Synthesis, Pergamon, Oxford, 2002, pp. 273–335.
10.1016/S1460-1567(02)80038-1 Google Scholar
- 13Disilyne 3 has the lowest molecular weight among the reported isolable disilynes. Formula weight of disilynes: 3 514, 1 a 940, 1 b 836, 1 c 864, 2 1306.
- 14Synthetic details, characterization, and molecular structures obtained by X-ray structural analysis of compounds 3, 4 a, 4 b, 6, 7, 8 a, and 8 b are described in Supporting Information. CCDC 957867 (3), 957868 (6), 957869 (7), 957870 (a single crystal of a 2:1 mixture of 8 a and 8 b), 957871 (4 a), 957872 (4 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15The 29Si resonance signals of 2 and 3 were considerably upfield shifted from symmetrically substituted disilyldisilynes (δ=91.5 ppm for 1 a and δ=89.9 ppm for 1 b).[1, 2] Magnetic anisotropic effect is suggested for the reason of the upfield shift.[4, 5]
- 16Assignments of the transitions based on the computational study of 3fix at the TD-B3LYP/SDD for Pd, 6-311+G(2df,p) for H, C, Si: band II is a mixed transition of πin→π*in and πout→π*out (HOMO−1 to LUMO and HOMO to LUMO+1); band III, πout→σ* (HOMO to LUMO+2); band IV, πout→π*out (HOMO to LUMO+1), respectively.
- 17A η1-disilenide zirconium complex, [(Cp2ZrCl)(Tip)SiSiTip2], showing a ligand-to-metal charge transfer absorption band at 715 nm has been reported, see: T.-I. Nguyen, D. Scheschkewitz, J. Am. Chem. Soc. 2005, 127, 10174–10175.
- 18The substituent effects of trialkylsilyl groups and alkyl groups on disilynes are verified by the theoretical studies using the optimized structures of the model compounds tBuSiSitBu (3 m) and Me3SiSiSiSiMe3 (1 m) at the B3LYP/6-31G(d) level. The smaller HOMO–LUMO gap of 3 m compared to that of 1 m was reproduced as shown in Figure S22 in Supporting Information. The πout and π*out orbitals of 3 m were higher in energy than those of 1 m owing to the less effective π–σ* interaction of alkyl groups compared to that of trialkylsilyl groups, whereas the energy levels of πin and π*in orbitals of 3 m are lower than those of 1 m because of more electronegative alkyl groups.
- 19For π–σ*(Si–C) and π*–σ*(Si–C) orbital interactions of silyl group, see; A. R. Bassindale, P. G. Taylor in The Chemistry of Organic Silicon Compounds, Vol. 1 (Eds.: ), Wiley, Chichester, 1989; pp. 893–963.
10.1002/0470025107.ch14 Google Scholar
- 20Several bi(silacyclopropane)s have been reported:
- 20aW. Ando, T. Shiba, T. Hidaka, K. Morihashi, O. Kikuchi, J. Am. Chem. Soc. 1997, 119, 3629–3630;
- 20bK. R. Pichaandi, J. T. Mague, M. J. Fink, J. Organomet. Chem. 2011, 696, 1957–1963.
- 21Recrystallization of a 1:1 mixture of 8 a and 8 b formed single crystals of 8 a and 8 b with a 2:1 ratio which was analyzed by X-ray diffraction study. As well as the reported bi(silacyclopropane) compounds, compounds 8 a and 8 b have short Si–Si bonds of 2.2928(11) Å and 2.3126(16) Å in comparison with the standard Si–Si single bond length (2.34 Å)[22] owing to the increased s character of the exocyclic Si–Si bond. Separation of 8 a and 8 b has not been possible.
- 22J. Y. Corey in The Chemistry of Organic Silicon Compounds, Vol. 1 (Eds.: ), Wiley, Chichester, 1989, pp. 1–56.
- 23S. Otsuka, T. Yoshida, M. Matsumoto, K. Nakatsu, J. Am. Chem. Soc. 1976, 98, 5850–5858.
- 24Elongation of silicon–silicon bond is calculated as Δr/r0=[(r−r0)/r0]×100 %, where r0 is the SiSi bond length in free disilyne 3. The bent-back angle is defined as an angle between a SiR bond axis and a plane passing through two silicon atoms and perpendicular to PdSiSi ring plane.
- 25
- 25aT. Iwamoto, C. Kabuto, M. Kira, J. Am. Chem. Soc. 1999, 121, 886–887;
- 25bM. Ichinohe, T. Matsuno, A. Sekiguchi, Angew. Chem. 1999, 111, 2331–2333;
10.1002/(SICI)1521-3757(19990802)111:15<2331::AID-ANGE2331>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2194–2196;10.1002/(SICI)1521-3773(19990802)38:15<2194::AID-ANIE2194>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 25cT. Iwamoto, M. Tamura, C. Kabuto, M. Kira, Science 2000, 290, 504–506;
- 25dM. Ichinohe, M. Igarashi, K. Sanuki, A. Sekiguchi, J. Am. Chem. Soc. 2005, 127, 9978–9979;
- 25eV. Y. Lee, H. Yasuda, A. Sekiguchi, J. Am. Chem. Soc. 2007, 129, 2436–2437;
- 25fK. Uchiyama, S. Nagendran, S. Ishida, T. Iwamoto, M. Kira, J. Am. Chem. Soc. 2007, 129, 10638–10639;
- 25gK. Leszczyńska, K. Abersfelder, A. Mix, B. Neumann, H. G. Stammler, M. J. Cowley, P. Jutzi, D. Scheschkewitz, Angew. Chem. 2012, 124, 6891–6895;
10.1002/ange.201202277 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6785–6788.
- 26E. K. Pham, R. West, Organometallics 1990, 9, 1517–1523.
- 27
- 27aM. J. S. Dewar, Bull. Soc. Chim. Fr. 1951, 18, C 71–C79;
- 27bJ. Chatt, L. A. Duncanson, J. Chem. Soc. 1953, 2939–2947. See also Ref. [8].
- 28Transition-metal η2-disilene complexes, see:
- 28aC. Zybill, R. West, J. Chem. Soc. Chem. Commun. 1986, 857–858;
- 28bE. K. Pham, R. West, J. Am. Chem. Soc. 1989, 111, 7667–7668;
- 28cD. H. Berry, J. H. Chey, H. S. Zipin, P. J. Carroll, J. Am. Chem. Soc. 1990, 112, 452–453;
- 28dD. H. Berry, J. H. Chey, H. S. Zipin, P. J. Carroll, Polyhedron 1991, 10, 1189–1201;
- 28eP. Hong, N. H. Damrauer, P. J. Carroll, D. H. Berry, Organometallics 1993, 12, 3698–3704;
- 28fH. Hashimoto, Y. Sekiguchi, T. Iwamoto, C. Kabuto, M. Kira, Organometallics 2002, 21, 454–456;
- 28gH. Hashimoto, Yo. Sekiguchi, Yu. Sekiguchi, T. Iwamoto, C. Kabuto, M. Kira, Can. J. Chem. 2003, 81, 1241–1245;
- 28hM. Kira, Y. Sekiguchi, T. Iwamoto, C. Kabuto, J. Am. Chem. Soc. 2004, 126, 12778–12779;
- 28iH. Hashimoto, K. Suzuki, W. Setaka, C. Kabuto, M. Kira, J. Am. Chem. Soc. 2004, 126, 13628–13629;
- 28jR. Fischer, M. Zirngast, M. Flock, J. Baumgartner, C. Marschner, J. Am. Chem. Soc. 2005, 127, 70–71;
- 28kT. Iwamoto, Y. Sekiguchi, N. Yoshida, C. Kabuto, M. Kira, Dalton Trans. 2006, 177–182;
- 28lT. Abe, T. Iwamoto, M. Kira, J. Am. Chem. Soc. 2010, 132, 5008–5009;
- 28mM. Hartmann, A. Haji-Abdi, K. Abersfelder, P. R. Haycock, A. J. P. White, D. Scheschkewitz, Dalton Trans. 2010, 39, 9288–9295.
- 29Destabilization by increasing orbital overlap between filled d and πin orbitals on changing from a trans-bent to planar structure may be also responsible for the trans-bent structures of the disilyne moiety in 4 a an 4 b.
- 30A Y-shaped structure with planar silicon–silicon double bond (4 m′′) was found as a second-order saddle point and to be 75.0 kJ mol−1 higher in energy than 4 m.