Palladium(II)-Catalyzed Cyclizative Cross-Coupling of ortho-Alkynylanilines with ortho-Alkynylbenzamides under Aerobic Conditions†
Dr. Bo Yao
Laboratory of Synthesis and Natural Product, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304, 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Search for more papers by this authorDr. Qian Wang
Laboratory of Synthesis and Natural Product, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304, 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Search for more papers by this authorCorresponding Author
Prof. Dr. Jieping Zhu
Laboratory of Synthesis and Natural Product, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304, 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Laboratory of Synthesis and Natural Product, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304, 1015 Lausanne (Switzerland) http://lspn.epfl.ch===Search for more papers by this authorDr. Bo Yao
Laboratory of Synthesis and Natural Product, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304, 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Search for more papers by this authorDr. Qian Wang
Laboratory of Synthesis and Natural Product, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304, 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Search for more papers by this authorCorresponding Author
Prof. Dr. Jieping Zhu
Laboratory of Synthesis and Natural Product, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304, 1015 Lausanne (Switzerland) http://lspn.epfl.ch
Laboratory of Synthesis and Natural Product, Ecole Polytechnique Fédérale de Lausanne, EPFL-SB-ISIC-LSPN, BCH 5304, 1015 Lausanne (Switzerland) http://lspn.epfl.ch===Search for more papers by this authorWe thank the EPFL (Switzerland), Swiss National Science Foundation (SNSF), and Swiss National Centers of Competence in Research NCCR Chemical Biology for financial support.
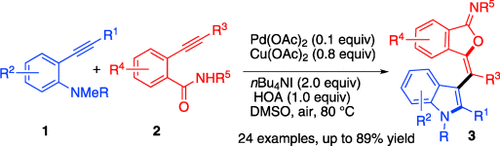
Graphical Abstract
Born to couple: The Pd(OAc)2-catalyzed reaction of o-alkynylanilines (1) with o-alkynylbenzamides (2) affords the cyclizative cross-coupling products 3 in good to excellent yields. Three bonds are created in the formation of two heterocycles tethered by a tetrasubstituted double bond. Mechanistic studies indicate that the reaction is initiated by aminopalladation with subsequent oxypalladation, N-demethylation, and reductive elimination.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201307738_sm_miscellaneous_information.pdf5.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. I. McDonald, G. Liu, S. S. Stahl, Chem. Rev. 2011, 111, 2981–3019.
- 2Cyclizative dimerization of alkynes. Gold-catalyzed, see:
- 2aM. G. Auzias, M. Neuburger, H. A. Wegner, Synlett 2010, 2443–2448;
- 2bH. A. Wegner, S. Ahles, M. Neuburger, Chem. Eur. J. 2008, 14, 11310–11313;
- 2cO. L. Egorova, H. Seo, Y. Kim, D. Moon, Y.-M. Rhee, K. H. Ahn, Angew. Chem. 2011, 123, 11648–11652;
10.1002/ange.201106132 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11446–11450;
- 2dE. Marchal, P. Uriac, B. Legouin, L. Toupet, P. van de Weghe, Tetrahedron 2007, 63, 9979–9990; Palladium-catalyzed:
- 2eN. Furuichi, H. Hara, T. Osaki, M. Nakano, H. Mori, S. Katsumura, J. Org. Chem. 2004, 69, 7949–7959;
- 2fB. Alcaide, P. Almendros, C. Aragoncillo, Chem. Eur. J. 2009, 15, 9987–9989;
- 2gS. Yasuhara, M. Sasa, T. Kusakabe, H. Takayama, M. Kimura, T. Mochida, K. Kato, Angew. Chem. 2011, 123, 3998–4001;
10.1002/ange.201008139 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 3912–3915;
- 2hB. Yao, C. Jaccoud, Q. Wang, J. Zhu, Chem. Eur. J. 2012, 18, 5864–5868.
- 3Cyclizative dimerization of allenes. Palladium-catalyzed:
- 3aS. Ma, Z. Yu, Angew. Chem. 2002, 114, 1853–1856;
Angew. Chem. Int. Ed. 2002, 41, 1775–1778;
10.1002/1521-3773(20020517)41:10<1775::AID-ANIE1775>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 3bS. Ma, Z. Yu, Z. Gu, Chem. Eur. J. 2005, 11, 2351–2356. Gold-catalyzed:
- 3cS. Ma, Z. Gu, Z. Yu, J. Org. Chem. 2005, 70, 6291–6294;
- 3dA. S. K. Hashmi, M. C. Blanco, D. Fischer, J. W. Bats, Eur. J. Org. Chem. 2006, 1387–1389.
- 4
- 4aY. Luo, L. Hong, J. Wu, Chem. Commun. 2011, 47, 5298–5300;
- 4bX. Pan, Y. Luo, J. Wu, Chem. Commun. 2011, 47, 8967–8969;
- 4cY. Luo, J. Wu, Chem. Commun. 2011, 47, 11137–11139;
- 4dY. Luo, X. Pan, J. Wu, Adv. Synth. Catal. 2012, 354, 3071–3077.
- 5
- 5aJ.-M. Weibel, A. Blanc, P. Pale, Chem. Rev. 2008, 108, 3149–3173;
- 5bS. F. Kirsch, Synthesis 2008, 3183–3204;
- 5cL.-N. Guo, X.-H. Duan, Y.-M. Liang, Acc. Chem. Res. 2011, 44, 111–122.
- 6Bis(indole) is a major side product in the optimization of cyclizative alkynylation reported in this paper: B. Yao, Q. Wang, J. Zhu, Angew. Chem. 2012, 124, 12477–12481; Angew. Chem. Int. Ed. 2012, 51, 12311–12315.
- 7
- 7aG. Zeni, R. C. Larock, Chem. Rev. 2004, 104, 2285–2309;
- 7bS. Cacchi, G. Fabrizi, Chem. Rev. 2011, 111, PR 215–283.
- 85-exo-dig cyclization:
- 8aY. Koseki, T. Nagasaka, Chem. Pharm. Bull. 1995, 43, 1604–1606;
- 8bM. W. Khan, N. G. Kundu, Synlett 1997, 1435–1437;
- 8cN. G. Kundu, M. W. Khan, Tetrahedron 2000, 56, 4777–4792;
- 8dC. Kanazawa, M. Terada, Chem. Asian J. 2009, 4, 1668–1672;
- 8eG. Bianchi, M. Chiarini, F. Marinelli, L. Rossi, A. Arcadi, Adv. Synth. Catal. 2010, 352, 136–142.
- 96-endo-dig cyclization:
- 9aT. L. Yao, R. C. Larock, J. Org. Chem. 2005, 70, 1432–1437;
- 9bN. Sakai, K. Annaka, A. Fujita, A. Sato, T. Konakahara, J. Org. Chem. 2008, 73, 4160–4165;
- 9cG. Liu, Y. Zhou, D. Ye, D. Zhang, X. Ding, H. Jiang, H. Liu, Adv. Synth. Catal. 2009, 351, 2605–2610;
- 9dM. Bian, W. J. Yao, H. F. Ding, C. Ma, J. Org. Chem. 2010, 75, 269–272.
- 10K. Gilmore, I. V. Alabugin, Chem. Rev. 2011, 111, 6513–6556.
- 11B. Yao, Q. Wang, J. Zhu, Angew. Chem. 2012, 124, 5260–5264; Angew. Chem. Int. Ed. 2012, 51, 5170–5174.
- 12C3-unsubstituted indoles were the major products if primary and secondary anilines were used as reaction partners.
- 13CCDC 958685 (3 ae) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 14Trapping of an σ-indolyl/PdII intermediate, see Refs. [6,11] and
- 14aA. Yasuhara, Y. Takeda, N. Suzuki, T. Sakamoto, Chem. Pharm. Bull. 2002, 50, 235–238;
- 14bX. Han, X. Lu, Org. Lett. 2010, 12, 3336–3339;
- 14cC. C. Chin, L.-Y. Chin, S.-C. Yang, M.-J. Wu, Org. Lett. 2010, 12, 5652–5655;
- 14dR. Álvarez, C. Martínez, Y. Madich, J. G. Denis, J. M. Aurrecoechea, Á. R. De Lera, Chem. Eur. J. 2010, 16, 12746–12753;
- 14eX.-F. Xia, N. Wang, L.-L. Zhang, X.-R. Song, X.-Y. Liu, Y.-M. Liang, J. Org. Chem. 2012, 77, 9163–9170;
- 14fZ. Hu, D. Liang, J. Zhao, J. Huang, Q. Zhu, Chem. Commun. 2012, 48, 7371–7373;
- 14gG. Qiu, C. Chen, L. Yao, J. Wu, Adv. Synth. Catal. 2013, 355, 1579–1584;
- 14hA. Arcadi, S. Cacchi, G. Fabrizi, A. Goggiamani, A. Lazzetti, F. Marinelli, Org. Biomol. Chem. 2013, 11, 545–548.
- 15A minor compound that matches the structure of A′ was detected in the NMR spectrum of the crude reaction mixture of this control experiment. However, we did not have convincing evidence to support this structural assignment.
- 16Reviews on palladium(II)-catalyzed oxidative transformation of alkenes and alkynes:
- 16asee Ref. [1];
- 16bW. Wu, H. Jiang, Acc. Chem. Res. 2012, 45, 1736–1748.