ChenPhos: Highly Modular P-Stereogenic C1-Symmetric Diphosphine Ligands for the Efficient Asymmetric Hydrogenation of α-Substituted Cinnamic Acids
Corresponding Author
Dr. Weiping Chen
Solvias AG, WRO-1055.6.68B, Mattenstrasse 22, Postfach, 4002 Basel (Switzerland)
Present address: School of Pharmacy, Fourth Military Medical University, 169 Changle West Road, Xi'an, 710032 (P. R. China)
Solvias AG, WRO-1055.6.68B, Mattenstrasse 22, Postfach, 4002 Basel (Switzerland)Search for more papers by this authorDr. Felix Spindler
Solvias AG, WRO-1055.6.68B, Mattenstrasse 22, Postfach, 4002 Basel (Switzerland)
Search for more papers by this authorDr. Benoit Pugin
Solvias AG, WRO-1055.6.68B, Mattenstrasse 22, Postfach, 4002 Basel (Switzerland)
Search for more papers by this authorDr. Ulrike Nettekoven
Solvias AG, WRO-1055.6.68B, Mattenstrasse 22, Postfach, 4002 Basel (Switzerland)
Search for more papers by this authorCorresponding Author
Dr. Weiping Chen
Solvias AG, WRO-1055.6.68B, Mattenstrasse 22, Postfach, 4002 Basel (Switzerland)
Present address: School of Pharmacy, Fourth Military Medical University, 169 Changle West Road, Xi'an, 710032 (P. R. China)
Solvias AG, WRO-1055.6.68B, Mattenstrasse 22, Postfach, 4002 Basel (Switzerland)Search for more papers by this authorDr. Felix Spindler
Solvias AG, WRO-1055.6.68B, Mattenstrasse 22, Postfach, 4002 Basel (Switzerland)
Search for more papers by this authorDr. Benoit Pugin
Solvias AG, WRO-1055.6.68B, Mattenstrasse 22, Postfach, 4002 Basel (Switzerland)
Search for more papers by this authorDr. Ulrike Nettekoven
Solvias AG, WRO-1055.6.68B, Mattenstrasse 22, Postfach, 4002 Basel (Switzerland)
Search for more papers by this authorGraphical Abstract
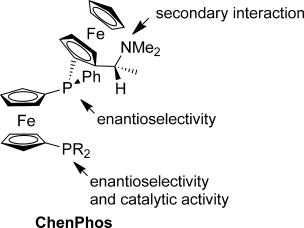
These cats are purrfectionists: The ChenPhos ligands (see structure) showed dramatically higher catalytic activity in the title reaction than their C2-symmetric predecessor with two dimethylaminoethyl-substituted ferrocenyl(phenyl)phosphanyl groups. The ready accessibility, extreme air stability, and high enantioselectivity, activity, and productivity of these ligands make them very promising for a wide range of practical applications.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201304472_sm_miscellaneous_information.pdf5.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For representative reviews, see:
- 1aW. Tang, X. Zhang, Chem. Rev. 2003, 103, 3029;
- 1bG Shang, W. Li, X. Zhang, Catalytic Asymmetric Synthesis (Ed.: ), 3rd ed., Wiley-VCH, Weinheim, 2010, p. 343;
- 1cJ. M. Brown, Comprehensive Asymmetric Catalysis (Eds.: ), Springer, Berlin, 1999, p. 121;
10.1007/978-3-642-58571-5 Google Scholar
- 1dK. V. L. Krépy, T. Imamoto, Top. Curr. Chem. 2003, 229, 1;
- 1eP. J. Guiry, C. P. Saunders, Adv. Synth. Catal. 2004, 346, 497.
- 2
- 2aH. B. Kagan, T.-P. Dang, J. Chem. Soc. Chem. Commun. 1971, 481;
- 2bH. B. Kagan, T.-P. Dang, J. Am. Chem. Soc. 1972, 94, 6429; diop=4,5-bis(diphenylphosphanylmethyl)-2,2-dimethyl-1,3-dioxalane.
- 3For reviews on the role of symmetry in asymmetric catalysis, see:
- 3aJ. K. Whitesell, Chem. Rev. 1989, 89, 1581;
- 3bC. Moberg, Isr. J. Chem. 2012, 52, 653; dipamp=1,2-ethanediylbis[(2-methoxyphenyl)phenylphosphane], binap=2,2′-bis(diphenylphosphanyl)-1,1′-binaphthyl, MeO-biphep=2,2′-bis(diphenylphosphanyl)-6,6′-dimethoxy-1,1′-biphenyl, Duphos=1,2-bis(2,5-dialkylphospholano)benzene.
- 4For representative examples of C2-symmetric diphosphine ligands, see:
- 4aG. Zhu, P. Cao, Q. Jiang, X. Zhang, J. Am. Chem. Soc. 1997, 119, 1799;
- 4bP. J. Pye, K. Rossen, R. A. Reamer, N. N. Tsou, R. P. Volante, P. J. Reider, J. Am. Chem. Soc. 1997, 119, 6207;
- 4cT. Imamoto, J. Watanabe, Y. Wada, H. Masuda, H. Yamada, H. Tsuruta, S. Matsukawa, K. Yamaguchi, J. Am. Chem. Soc. 1998, 120, 1635;
- 4dC.-C. Pai, C.-W. Lin, C.-C. Lin, C.-C. Chen, A. S. C. Chan, W. T. Wong, J. Am. Chem. Soc. 2000, 122, 11513;
- 4eD. Xiao, X. Zhang, Angew. Chem. 2001, 113, 3533;
Angew. Chem. Int. Ed. 2001, 40, 3425;
10.1002/1521-3773(20010917)40:18<3425::AID-ANIE3425>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar
- 4fT. Saito, T. Yokoawa, T. Ishizakik, T. Moroi, N. Sayo, T. Miura, H. Kumobayashi, Adv. Synth. Catal. 2001, 343, 264;
- 4gW. Tang, X. Zhang, Angew. Chem. 2002, 114, 1682;
Angew. Chem. Int. Ed. 2002, 41, 1612;
10.1002/1521-3773(20020503)41:9<1612::AID-ANIE1612>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 4hC.-C. Pai, Y.-M. Li, Z.-Y. Zhou, A. S. C. Chan, Tetrahedron Lett. 2002, 43, 2789;
- 4iL. Qiu, J. Qi, C.-C. Pai, S.-S. Chan, Z.-Y. Zhou, M. C. K. Choi, A. S. C. Chan, Org. Lett. 2002, 4, 4599;
- 4jJ.-H. Xie, L.-X. Wang, Y. Fu, S.-F. Zhu, B.-M. Fan, H.-F. Duan, Q.-L. Zhou, J. Am. Chem. Soc. 2003, 125, 4404;
- 4kS. D. de Paule, S. Jeulin, V. Ratovelomanana-Vidal, J.-P. Genêt, N. Champion, P. Dellis, Eur. J. Org. Chem. 2003, 1931;
- 4lL. Qiu, J. Wu, S.-S. Chan, T. T.-L. Au-Yeung, J.-X. Ji, R. W. Guo, C.-C. Pai, Z.-Y. Zhou, X. S. Li, Q. H. Fan, A. S. C. Chan, Proc. Natl. Acad. Sci. USA 2004, 101, 5815;
- 4mS. Jeulin, S. Duprat de Paule, V. Ratovelomanana-Vidal, J.-P. Genêt, N. Champion, Angew. Chem. 2004, 116, 324;
10.1002/ange.200352453 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 320;
- 4nS. Wu, W. Zhang, Z. Zhang, X. Zhang, Org. Lett. 2004, 6, 3565;
- 4oY. Sun, X. Wan, M. Guo, D. Wang, X. Dong, Y. Pan, Z. Zhang, Tetrahedron: Asymmetry 2004, 15, 2185;
- 4pX.-P. Hu, Z. Zheng, Org. Lett. 2004, 6, 3585;
- 4qT. Imamoto, N. Oohara, H. Takahashi, Synthesis 2004, 1353;
- 4rX. Cheng, Q. Zhang, J.-H. Xie, L.-X. Wang, Q.-L. Zhou, Angew. Chem. 2005, 117, 1142; Angew. Chem. Int. Ed. 2005, 44, 1118;
- 4sD. Liu, X. Zhang, Eur. J. Org. Chem. 2005, 646;
- 4tT. Imamoto, K. Sugita, K. Yoshida, J. Am. Chem. Soc. 2005, 127, 11934;
- 4uM. Kesselgruber, M. Lotz, P. Martin, G. Melone, M. Müller, B. Pugin, F. Naud, F. Spindler, M. Thommen, P. Zbinden, H.-U. Blaser, Chem. Asian J. 2008, 3, 1384;
- 4vX. Sun, L. Zhou, W. Li, X. Zhang, J. Org. Chem. 2008, 73, 1143;
- 4wX. Zhang, K. Huang, G. Hou, B. Cao, X. Zhang, Angew. Chem. 2010, 122, 6565; Angew. Chem. Int. Ed. 2010, 49, 6421;
- 4xW. Tang, B. Qu, A. G. Capacci, S. Rodriguez, X. Wei, N. Haddad, B. Narayanan, S. Ma, N. Grinberg, N. K. Yee, D. Krishnamurthy, C. H. Senanayake, Org. Lett. 2010, 12, 176;
- 4yK. Tamura, M. Sugiya, K. Yoshida, A. Yanagisawa, T. Imamoto, Org. Lett. 2010, 12, 4400;
- 4zT. Imamoto, K. Tamura, Z. Zhang, Y. Horiuchi, M. Sugiya, K. Yoshida, A. Yanagisawa, I. D. Gridnev, J. Am. Chem. Soc. 2012, 134, 1754;
- 4aaS. Doherty, C. H. Smyth, Nat. Protoc. 2012, 7, 1884.
- 5T. Ohkuma, M. Kitamura, R. Noyori in Catalytic Asymmetric Synthesis (Ed.: ), 2nd ed. Wiley-VCH, New York, 2000, pp. 1–110.
10.1002/0471721506.ch1 Google Scholar
- 6W. Chen, P. J. McCormack, K. Mohammed, W. Mbafor, S. M. Roberts, J. Whittall, Angew. Chem. 2007, 119, 4219; Angew. Chem. Int. Ed. 2007, 46, 4141.
- 7H.-U. Blaser, B. Pugin, F. Spindler, J. Mol. Catal. A 2005, 231, 1.
- 8ChenPhos is a trademark name for 1-[2-(1-N,N-dimethylaminoethyl)ferrocen-1-yl]aryl- (or alkyl) phosphanyl-1′-dialkyl- (or aryl) phosphanylferrocene ligands. They are available from Solvias AG, and some are listed in the catalogues of Aldrich and Strem.
- 9For reviews on C2- versus C1-symmetric ligands, see:
- 9aG. Helmchen, A. Pfaltz, Acc. Chem. Res. 2000, 33, 336;
- 9bA. Pfaltz, W. J. Drury III, Proc. Natl. Acad. Sci. USA 2004, 101, 5723;
- 9cV. A. Pavlov, Tetrahedron 2008, 64, 1147.
- 10K. Inoguchi, S. Sakuraba, K. Achiwa, Synlett 1992, 169.
- 11It is not unusual for a C1-symmetric ligand to give better results than the corresponding C2-symmetric ligand. For C1-symmetric 1,2-bis(alkylmethylphosphanyl)ethane (BisP*) ligands, see:
- 11aA. Ohashi, T. Imamoto, Tetrahedron Lett. 2001, 42, 1099;
- 11bA. Ohashi, T. Imamoto, Org. Lett. 2001, 3, 373;
- 11cA. Ohashi, S. Kikuchi, M. Yasutake, T. Imamoto, Eur. J. Org. Chem. 2002, 2535;
- 11dA. Ohashi, Chirality 2002, 14, 573; for C1-symmetric binap, see:
- 11eS. Gladiali, S. Pulacchini, D. Fabbri, M. Manassero, M. Sansoni, Tetrahedron: Asymmetry 1998, 9, 391;
- 11fS. Gladiali, A. Dore, D. Fabbri, S. Medici, G. Pirri, S. Pulacchini, Eur. J. Org. Chem. 2000, 2861;
- 11gT. Hayashi, T. Senda, Y. Takaya, M. Ogasawara, J. Am. Chem. Soc. 1999, 121, 11591; for C1-symmetric DuPhos, see:
- 11hK. W. Kottsieper, U. Kühner, O. Stelzer, Tetrahedron: Asymmetry 2001, 12, 1159;
- 11iK. Matsumura, H. Shimizu, T. Saito, H. Kumobayashi, Adv. Synth. Catal. 2003, 345, 180; for C1-symmetric 2,2′-bis(diphenylphosphanyl)-6,6′-dimethyl-1,1′-biphenyl (biphemp) and MeO-biphep, see:
- 11jR. Schmid, E. A. Broger, M. Cereghetti, Y. Crameri, J. Foricher, M. Lalonde, R. K. Müller, M. Scalone, G. Schoettel, U. Zutter, Pure Appl. Chem. 1996, 68, 131, and references therein; for C1-symmetric diop, see:
- 11kM. Chiba, H. Takahashi, T. Morimota, K. Achiwa, Tetrahedron Lett. 1987, 28, 3675;
- 11lT. Morimoto, M. Chiba, K. Achiwa, Tetrahedron Lett. 1988, 29, 4755.
- 12For examples of the design of C1-symmetric ligands, see:
- 12aG. Hoge, H.-P. Wu, W. S. Kissel, D. A. Pflum, D. J. Greene, J. Bao, J. Am. Chem. Soc. 2004, 126, 5966;
- 12bH.-P. Wu, G. Hoge, Org. Lett. 2004, 6, 3645;
- 12cF. Leroux, H. Mettler, Synlett 2006, 766;
- 12dF. R. Leroux, H. Mettler, Adv. Synth. Catal. 2007, 349, 323;
- 12eQ. Dai, W. Li, X. Zhang, Tetrahedron 2008, 64, 6943;
- 12fW. Tang, A. G. Capacci, A. White, S. Ma, S. Rodriguez, B. Qu, J. Savoie, N. D. Patel, X. Wei, N. Haddad, N. Grinberg, N. K. Yee, D. Krishnamurthy, C. H. Senanayake, Org. Lett. 2010, 12, 1104;
- 12gK. Huang, X. Zhang, T. J. Emge, G. Hou, B. Cao, X. Zhang, Chem. Commun. 2010, 46, 8555;
- 12hL. Bonnafoux, R. Gramage-Doria, F. Colobert, F. R. Leroux, Chem. Eur. J. 2011, 17, 11008.
- 13
- 13aW. Chen, W. Mbafor, S. M. Roberts, J. Whittall, J. Am. Chem. Soc. 2006, 128, 3922;
- 13bW. Chen, S. M. Roberts, J. Whittall, A. Steiner, Chem. Commun. 2006, 2916.
- 14Thermal epimerization through pyramidal inversion has been underutilized in the practical synthesis of P-chiral phosphines; for some examples, see:
- 14aA. Bader, M. Pabel, S. B. Wild, J. Chem. Soc. Chem. Commun. 1994, 1405;
- 14bM. Pabel, A. C. Willis, S. B. Wild, Inorg. Chem. 1996, 35, 1244;
- 14cA. Bader, M. Pabel, A. C. Willis, S. B. Wild, Inorg. Chem. 1996, 35, 3874;
- 14dE. Vedejs, Y. Donde, J. Org. Chem. 2000, 65, 2337;
- 14eG. Hoge, J. Am. Chem. Soc. 2004, 126, 9920.
- 15J. E. Frampton, M. P. Curran, Drugs 2007, 67, 1767.
- 16For the asymmetric hydrogenation of (E)-2-(3-(3-methoxypropoxy)-4-methoxybenzylidene)-3-methylbutanoic acid (7), see:
- 16aT. Sturm, W. Weissensteiner, F. Spindler, Adv. Synth. Catal. 2003, 345, 160;
- 16bJ. A. F. Boogers, U. Felfer, M. Kotthaus, L. Lefort, G. Steinbauer, A. H. M. De Vries, J. G. De Vries, Org. Process Res. Dev. 2007, 11, 585;
- 16cS. Li, S.-F. Zhu, C.-M. Zhang, S. Song, Q.-L. Zhou, J. Am. Chem. Soc. 2008, 130, 8584.
- 17It was not possible to purify ligand 1 c (R=tBu) by either crystallization or chromatography, and crude 1 c (ca. 90 % purity, as determined by 31P NMR spectroscopy) was used in the hydrogenation.