Friedel–Crafts Benzylation of Activated and Deactivated Arenes†
Gabriel Schäfer
Laboratorium für Organische Chemie, Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH) Zürich, Wolfgang Pauli Strasse 10, 8093 Zürich (Switzerland) http://www.bodegroup.org
Search for more papers by this authorCorresponding Author
Prof. Dr. Jeffrey W. Bode
Laboratorium für Organische Chemie, Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH) Zürich, Wolfgang Pauli Strasse 10, 8093 Zürich (Switzerland) http://www.bodegroup.org
Laboratorium für Organische Chemie, Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH) Zürich, Wolfgang Pauli Strasse 10, 8093 Zürich (Switzerland) http://www.bodegroup.orgSearch for more papers by this authorGabriel Schäfer
Laboratorium für Organische Chemie, Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH) Zürich, Wolfgang Pauli Strasse 10, 8093 Zürich (Switzerland) http://www.bodegroup.org
Search for more papers by this authorCorresponding Author
Prof. Dr. Jeffrey W. Bode
Laboratorium für Organische Chemie, Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH) Zürich, Wolfgang Pauli Strasse 10, 8093 Zürich (Switzerland) http://www.bodegroup.org
Laboratorium für Organische Chemie, Department of Chemistry and Applied Biosciences, Swiss Federal Institute of Technology (ETH) Zürich, Wolfgang Pauli Strasse 10, 8093 Zürich (Switzerland) http://www.bodegroup.orgSearch for more papers by this authorThis work was supported by ETH Research Grant ETH-12 11-1. We thank the ETH Zürich Mass Spectrometry Service for spectroscopic data, Aaron Dumas for helpful discussions, and Cam-Van Thi Vo for a preliminary study.
Graphical Abstract
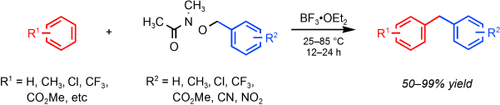
NO going back makes possible facile Friedel–Crafts benzylations with moderate reaction temperatures, simple reaction workups, and improved substrate scope for the formation of synthetically important diarylmethanes (see scheme). Upon complexation with BF3⋅OEt2, hydroxamates serve as reversible leaving groups that stabilize highly reactive carbocations. Even deactivated arenes and electron-deficient benzylhydroxamates react cleanly under these conditions.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201105380_sm_miscellaneous_information.pdf3.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. Friedel, J. M. Crafts, C. R. Hebd. Seances Acad. Sci. 1877, 84, 1450;
- 1bR. M. Roberts, A. A. Khalaf, Friedel–Crafts Alkylation Chemistry, Marcel Dekker, New York, 1984;
- 1cG. A. Olah, R. Krishnamurti, G. K. S. Prakash in Comprehensive Organic Synthesis, Vol. 3 (Eds.: ), Pergamon, Oxford, 1991, pp. 293–339.
10.1016/B978-0-08-052349-1.00065-2 Google Scholar
- 2For recent reviews on transition-metal catalyzed CH functionalization, see:
- 2aX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. 2009, 121, 5196–5217; Angew. Chem. Int. Ed. 2009, 48, 5094–5115;
- 2bT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147–1169; for recent reviews on transition-metal catalyzed direct benzylation, see:
- 2cB. Liégault, J.-L. Renaud, C. Bruneau, Chem. Soc. Rev. 2008, 37, 290–299;
- 2dL. Ackermann, Chem. Commun. 2010, 46, 4866–4877.
- 3For other benzylation approaches, see:
- 3aY.-H. Chen, M. Sun, P. Knochel, Angew. Chem. 2009, 121, 2270–2273; Angew. Chem. Int. Ed. 2009, 48, 2236–2239;
- 3bH. Duan, L. Meng, D. Bao, H. Zhang, Y. Li, A. Lei, Angew. Chem. 2010, 122, 6531–6534; Angew. Chem. Int. Ed. 2010, 49, 6387–6390;
- 3cJ. Barluenga, M. Tomás-Gamasa, F. Aznar, C. Valdés, Nat. Chem. 2009, 1, 494–499.
- 4D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. L. Leazer, J. Russell, R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaksh, T. Zhang, Green Chem. 2007, 9, 411–420.
- 5For examples of direct meta-selective reactions, see:
- 5aH. A. Duong, R. E. Gilligan, M. L. Cooke, R. J. Phipps, M. J. Gaunt, Angew. Chem. 2011, 123, 483–486; Angew. Chem. Int. Ed. 2011, 50, 463–466;
- 5bY. H. Zhang, B. F. Shi, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 5072–5074;
- 5cJ. M. Murphy, X. Liao, J. F. Hartwig, J. Am. Chem. Soc. 2007, 129, 15434–15435; for a recent highlight article, see:
- 5dY. Zhou, J. Zhao, L. Liu, Angew. Chem. 2009, 121, 7262–7264; Angew. Chem. Int. Ed. 2009, 48, 7126–7128.
- 6For a recent review on new developments in Friedel–Crafts alkylation, see: M. Rueping, B. J. Nachtsheim, Beilstein J. Org. Chem. 2010, 6, 6.
- 7T. Yamauchi, K. Hattori, S. Mizutaki, K. Tamaki, S. Uemura, Bull. Chem. Soc. Jpn. 1986, 59, 3617–3620.
- 8T. Tsuchimoto, K. Tobita, T. Hiyama, S.-I. Fukuzawa, J. Org. Chem. 1997, 62, 6997–7005.
- 9I. Iovel, K. Mertins, J. Kischel, A. Zapf, M. Beller, Angew. Chem. 2005, 117, 3981–3985;
10.1002/ange.200462522 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3913–3917.
- 10M. Rueping, B. J. Nachtsheim, W. Ieawsuwan, Adv. Synth. Catal. 2006, 348, 1033–1037.
- 11K. Mertins, I. Iovel, J. Kischel, A. Zapf, M. Beller, Adv. Synth. Catal. 2006, 348, 691–695.
- 12S. Dasgupta, B. Török, Curr. Org. Synth. 2008, 5, 321–342.
- 13For nomenclature and chemistry of hydroxamic acids, see: L. Bauer, O. Exner, Angew. Chem. 1974, 86, 419–428; Angew. Chem. Int. Ed. Engl. 1974, 13, 376–384.
- 14
- 14aC.-V. Vo, T. A. Mitchell, J. W. Bode, J. Am. Chem. Soc. 2011, 133, 14082–14089;
- 14bT. A. Mitchell, J. W. Bode, J. Am. Chem. Soc. 2009, 131, 18057–18059.
- 15The starting hydroxamates are easily prepared from the corresponding benzylic chlorides and N-methyl hydroxamic acid. They are stable, easily handled materials. See the Supporting Information for their preparation and full characterization.
- 16FeCl3 proved completely ineffective for the arylation of deactivated hydroxamate substrates and for the benzylation of electron-poor arenes, even at elevated temperatures.
- 17The terms “activated” and “deactivated” refer to the relative nucleophilicity of the arenes compared to that of benzene. For a detailed review about π-nucleophilicity in carbon–carbon bond-forming, see: H. Mayr, B. Kempf, A. R. Ofial, Acc. Chem. Res. 2003, 36, 66–77.
- 18M. C. Wilkinson, Org. Lett. 2011, 13, 2232–2235.
- 19B. N. Campbell, E. C. Spaeth, J. Am. Chem. Soc. 1959, 81, 5933–5936.
- 20S. M. Nobre, A. L. Monteiro, Tetrahedron Lett. 2004, 45, 8225–8228.