Synthesis of 1-Phospha-2-boraacenaphthenes: Reductive 1,2-Aryl Migration of 1-Diarylboryl-8-dichlorophosphinonaphthalenes†
Dr. Akihiro Tsurusaki
Institute for Chemical Research, Kyoto University, Gakasho, Uji, Kyoto 611-0011 (Japan) http://boc.kuicr.kyoto-u.ac.jp/www/index-e.html
Search for more papers by this authorCorresponding Author
Prof. Dr. Takahiro Sasamori
Institute for Chemical Research, Kyoto University, Gakasho, Uji, Kyoto 611-0011 (Japan) http://boc.kuicr.kyoto-u.ac.jp/www/index-e.html
Institute for Chemical Research, Kyoto University, Gakasho, Uji, Kyoto 611-0011 (Japan) http://boc.kuicr.kyoto-u.ac.jp/www/index-e.htmlSearch for more papers by this authorProf. Dr. Atsushi Wakamiya
Department of Chemistry, Graduate School of Science, Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorProf. Dr. Shigehiro Yamaguchi
Department of Chemistry, Graduate School of Science, Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorKazuhiko Nagura
Department of Chemistry, Graduate School of Science, Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorProf. Dr. Stephan Irle
Department of Chemistry, Graduate School of Science, Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Norihiro Tokitoh
Institute for Chemical Research, Kyoto University, Gakasho, Uji, Kyoto 611-0011 (Japan) http://boc.kuicr.kyoto-u.ac.jp/www/index-e.html
Institute for Chemical Research, Kyoto University, Gakasho, Uji, Kyoto 611-0011 (Japan) http://boc.kuicr.kyoto-u.ac.jp/www/index-e.htmlSearch for more papers by this authorDr. Akihiro Tsurusaki
Institute for Chemical Research, Kyoto University, Gakasho, Uji, Kyoto 611-0011 (Japan) http://boc.kuicr.kyoto-u.ac.jp/www/index-e.html
Search for more papers by this authorCorresponding Author
Prof. Dr. Takahiro Sasamori
Institute for Chemical Research, Kyoto University, Gakasho, Uji, Kyoto 611-0011 (Japan) http://boc.kuicr.kyoto-u.ac.jp/www/index-e.html
Institute for Chemical Research, Kyoto University, Gakasho, Uji, Kyoto 611-0011 (Japan) http://boc.kuicr.kyoto-u.ac.jp/www/index-e.htmlSearch for more papers by this authorProf. Dr. Atsushi Wakamiya
Department of Chemistry, Graduate School of Science, Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorProf. Dr. Shigehiro Yamaguchi
Department of Chemistry, Graduate School of Science, Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorKazuhiko Nagura
Department of Chemistry, Graduate School of Science, Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorProf. Dr. Stephan Irle
Department of Chemistry, Graduate School of Science, Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Norihiro Tokitoh
Institute for Chemical Research, Kyoto University, Gakasho, Uji, Kyoto 611-0011 (Japan) http://boc.kuicr.kyoto-u.ac.jp/www/index-e.html
Institute for Chemical Research, Kyoto University, Gakasho, Uji, Kyoto 611-0011 (Japan) http://boc.kuicr.kyoto-u.ac.jp/www/index-e.htmlSearch for more papers by this authorThis work was partially supported by Grants-in-Aid for Creative Scientific Research (B) (No. 22350017), the Global COE Program B09, and the MEXT Project of Integrated Research on Chemical Synthesis from the Ministry of Education, Culture, Sports, Science and Technology (Japan).
Graphical Abstract
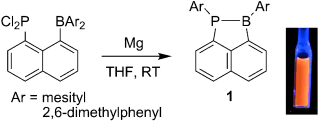
On the move: The title compounds 1, which are unique heterocyclic systems containing a PB bond, have been synthesized by the reduction of 1-diarylboryl-8-dichlorophosphinonaphthalene derivatives (see scheme). Both experimental and theoretical results for 1 revealed an effective interaction between the phosphorus atom, boron atom, and naphthyl moiety. Furthermore, 1 exhibits orange fluorescence in solution.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201104971_sm_miscellaneous_information.pdf632.4 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews of π-conjugated compounds containing a phosphorus atom, see:
- 1aT. Baumgartner, R. Réau, Chem. Rev. 2006, 106, 4681;
- 1bJ. Crassous, R. Réau, Dalton Trans. 2008, 6865.
- 2For reviews of π-conjugated compounds containing a boron atom, see:
- 2aC. D. Entwistle, T. B. Marder, Angew. Chem. 2002, 114, 3051;
10.1002/1521-3757(20020816)114:16<3051::AID-ANGE3051>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2927;10.1002/1521-3773(20020816)41:16<2927::AID-ANIE2927>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 2bC. D. Entwistle, T. B. Marder, Chem. Mater. 2004, 16, 4574;
- 2cS. Yamaguchi, A. Wakamiya, Pure Appl. Chem. 2006, 78, 1413;
- 2dF. Jäkle, Chem. Rev. 2010, 110, 3985.
- 3For a review of π-conjugated systems with BN bond, see:
- 3aM. J. D. Bosdet, W. E. Piers, Can. J. Chem. 2009, 87, 8; for recent progress in π-electron conjugated systems:
- 3bM. Lepeltier, O. Lukoyanova, A. Jacobson, S. Jeeva, D. F. Perepichka, Chem. Commun. 2010, 46, 7007;
- 3cT. Taniguchi, S. Yamaguchi, Organometallics 2010, 29, 5732.
- 4
- 4aT. Agou, J. Kobayashi, T. Kawashima, Org. Lett. 2005, 7, 4373;
- 4bT. Agou, J. Kobayashi, T. Kawashima, Inorg. Chem. 2006, 45, 9137.
- 5
- 5aJ. Yoshino, N. Kano, T. Kawashima, Chem. Commun. 2007, 559;
- 5bJ. Yoshino, N. Kano, T. Kawashima, Chem. Lett. 2008, 37, 960.
- 6A. Wakamiya, K. Mori, T. Araki, S. Yamaguchi, J. Am. Chem. Soc. 2009, 131, 10850.
- 7For a review, see:
- 7aA. Fukazawa, S. Yamaguchi, Chem. Asian J. 2009, 4, 1386; for examples of phosphonium- and borate-bridging zwitterionic stilbenes, see:
- 7bA. Fukazawa, H. Yamada, S. Yamaguchi, Angew. Chem. 2008, 120, 5664;
10.1002/ange.200801834 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5582;
- 7cA. Fukazawa, H. Yamada, Y. Sasaki, S. Akiyama, S. Yamaguchi, Chem. Asian J. 2010, 5, 466.
- 8For reviews, see:
- 8aR. T. Paine, H. Nöth, Chem. Rev. 1995, 95, 343;
- 8bP. P. Power, Chem. Rev. 1999, 99, 3463;
- 8cR. C. Fischer, P. P. Power, Chem. Rev. 2010, 110, 3877.
- 9
- 9aT. L. Allen, A. C. Scheiner, H. F. Schaefer III, Inorg. Chem. 1990, 29, 1930;
- 9bM. B. Coolidge, W. T. Borden, J. Am. Chem. Soc. 1990, 112, 1704;
- 9cT. L. Allen, W. H. Fink, Inorg. Chem. 1992, 31, 1703;
- 9dT. L. Allen, W. H. Fink, Inorg. Chem. 1993, 32, 4230;
- 9eH.-J. Himmel, Dalton Trans. 2003, 3639;
- 9fD. J. Grant, D. A. Dixon, J. Phys. Chem. A 2006, 110, 12955;
- 9gT. M. Gilbert, S. M. Bachrach, Organometallics 2007, 26, 2672.
- 10A. Staubitz, A. P. M. Robertson, M. E. Sloan, I. Manners, Chem. Rev. 2010, 110, 4023.
- 11
- 11aD. C. Pestana, P. P. Power, J. Am. Chem. Soc. 1991, 113, 8426;
- 11bX. Feng, M. M. Olmstead, P. P. Power, Inorg. Chem. 1986, 25, 4615.
- 12
- 12aU. Vogel, A. Y. Timoshkin, K.-C. Schwan, M. Bodensteiner, M. Scheer, J. Organomet. Chem. 2006, 691, 4556;
- 12bK.-C. Schwan, A. Y. Timoskin, M. Zabel, M. Scheer, Chem. Eur. J. 2006, 12, 4900;
- 12cU. Vogel, P. Hoemensch, K.-C. Schwan, A. Y. Timoshkin, M. Scheer, Chem. Eur. J. 2003, 9, 515;
- 12dA. Adolf, U. Vogel, M. Zabel, A. Y. Timoshkin, M. Scheer, Eur. J. Inorg. Chem. 2008, 3482;
- 12eA. Adolf, M. Zabel, M. Scheer, Eur. J. Inorg. Chem. 2007, 2136.
- 13Although there are a few examples on the synthesis and complexation of heteroatom-substituted acenaphthene derivatives, their properties have not been studied. For examples, see:
- 13aR. Köster, G. Benedikt, W. Fenzl, K. Reinert, Justus Liebigs Ann. Chem. 1967, 702, 197;
- 13bM. Pailer, H. Huemer, Monatsh. Chem. 1964, 95, 373;
- 13cB. J. Anderson, M. A. Guino-o, D. S. Glueck, J. A. Golen, A. G. DiPasquale, L. M. Liable-Sands, A. L. Rheingold, Org. Lett. 2008, 10, 4425;
- 13dT. Mizuta, T. Nakazono, K. Miyoshi, Angew. Chem. 2002, 114, 4053;
10.1002/1521-3757(20021018)114:20<4053::AID-ANGE4053>3.0.CO;2-X Google ScholarAngew. Chem. Int. Ed. 2002, 41, 3897.10.1002/1521-3773(20021018)41:20<3897::AID-ANIE3897>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 14See the Supporting Information for details.
- 15The 1H NMR spectra of the crude mixture showed the signals of (E)-4 c and (Z)-4 c in the ratio of 7:10.
- 16Bertrand and co-workers have reported P2B2 four-membered-ring systems with BB bonds. For examples, see;
- 16aD. Scheschkewitz, H. Amii, H. Gornitzka, W. W. Schoeller, D. Bourissou, G. Bertrand, Science 2002, 295, 1880;
- 16bD. Scheschkewitz, H. Amii, H. Gornitzka, W. W. Schoeller, D. Bourissou, G. Bertrand, Angew. Chem. 2004, 116, 595;
10.1002/ange.200352944 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 585.
- 17CCDC 832939 (1 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 18To the best of our knowledge, there has been no report on the electrochemical and photophysical properties of phosphinoboranes.
- 19The reduction potentials of NaphBMes2 were previously reported as E1/2=−2.41 V and Epc=−3.36 V, see: J. D. Hoefelmeyer, S. Solé, F. P. Gabbaï, Dalton Trans. 2004, 1254.
- 20The broad absorption was observed around 280–400 nm (λmax=334 nm; ε, 1.4×103) in the UV/Vis spectrum of 2 a in hexanes.
- 21T. A. Smith, D. J. Haines, K. P. Ghiggino, J. Fluoresc. 2000, 10, 365, and references therein.
- 22No emission was observed using the excitation wavelengths at every 10 nm from 280 to 370 nm.
- 23Both kr and knr values of acenaphthene are calculated as 1.2×107 s−1 (quantum yield, 0.50; lifetime, 46 ns in cyclohexane), see: M. Montalti, A. Credi, L. Prodi, M. T. Gandolfi in Handbook of Photochemistry, 3rd. ed., CRC, Boca Raton, 2006, p. 86.
10.1201/9781420015195 Google Scholar
- 24
- 24aR. Ahlrichs, M. Bar, M. Haser, H. Horn, C. Kolmel, Chem. Phys. Lett. 1989, 162, 165;
- 24bTURBOMOLE User’s Manual, Version 6.2; R. Ahlrichs, F. Furche, C. Hättig, W. Klopper, M. Sierka, F. Weigend, TURBOMOLE ver. 6.2, 2010.
- 25The emission is most likely interpreted in terms of the intramolecular charge transfer (HOMO–LUMO) with a small change of the polarity at the excited state.