Click Syntheses of 1,2,3-Triazolylbiferrocenyl Dendrimers and the Selective Roles of the Inner and Outer Ferrocenyl Groups in the Redox Recognition of ATP2− and Pd2+†
Rodrigue Djeda
Institut des Sciences Moléculaires, UMR CNRS N°5255, Université Bordeaux 1, 33405 Talence Cedex (France), Fax: (+33) 5-4000-2995 http://astruc.didier.free.fr
Search for more papers by this authorAmalia Rapakousiou
Institut des Sciences Moléculaires, UMR CNRS N°5255, Université Bordeaux 1, 33405 Talence Cedex (France), Fax: (+33) 5-4000-2995 http://astruc.didier.free.fr
Search for more papers by this authorLiyuan Liang
Institut des Sciences Moléculaires, UMR CNRS N°5255, Université Bordeaux 1, 33405 Talence Cedex (France), Fax: (+33) 5-4000-2995 http://astruc.didier.free.fr
Search for more papers by this authorNicola Guidolin
Laboratoire de Chimie des Polymères Organiques, UMR CNRS N° 5629, Université Bordeaux 1, 33607 Pessac Cedex (France)
Search for more papers by this authorDr. Jaime Ruiz
Institut des Sciences Moléculaires, UMR CNRS N°5255, Université Bordeaux 1, 33405 Talence Cedex (France), Fax: (+33) 5-4000-2995 http://astruc.didier.free.fr
Search for more papers by this authorProf. Didier Astruc
Institut des Sciences Moléculaires, UMR CNRS N°5255, Université Bordeaux 1, 33405 Talence Cedex (France), Fax: (+33) 5-4000-2995 http://astruc.didier.free.fr
Search for more papers by this authorRodrigue Djeda
Institut des Sciences Moléculaires, UMR CNRS N°5255, Université Bordeaux 1, 33405 Talence Cedex (France), Fax: (+33) 5-4000-2995 http://astruc.didier.free.fr
Search for more papers by this authorAmalia Rapakousiou
Institut des Sciences Moléculaires, UMR CNRS N°5255, Université Bordeaux 1, 33405 Talence Cedex (France), Fax: (+33) 5-4000-2995 http://astruc.didier.free.fr
Search for more papers by this authorLiyuan Liang
Institut des Sciences Moléculaires, UMR CNRS N°5255, Université Bordeaux 1, 33405 Talence Cedex (France), Fax: (+33) 5-4000-2995 http://astruc.didier.free.fr
Search for more papers by this authorNicola Guidolin
Laboratoire de Chimie des Polymères Organiques, UMR CNRS N° 5629, Université Bordeaux 1, 33607 Pessac Cedex (France)
Search for more papers by this authorDr. Jaime Ruiz
Institut des Sciences Moléculaires, UMR CNRS N°5255, Université Bordeaux 1, 33405 Talence Cedex (France), Fax: (+33) 5-4000-2995 http://astruc.didier.free.fr
Search for more papers by this authorProf. Didier Astruc
Institut des Sciences Moléculaires, UMR CNRS N°5255, Université Bordeaux 1, 33405 Talence Cedex (France), Fax: (+33) 5-4000-2995 http://astruc.didier.free.fr
Search for more papers by this authorHelpful assistance and discussion of Mössbauer data with Prof. Azzedine Bousseksou (LCC, Toulouse), EPR data with Mattieu Duttine (ICMCB, Pessac), and syntheses with Dr. A. K. Diallo (ISM), and financial support from the Université Bordeaux I, the CNRS, and the ANR are gratefully acknowledged.
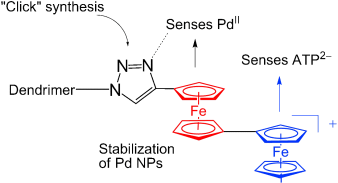
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201004756_sm_miscellaneous_information.pdf2.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on Fc dendrimers and their electrochemical properties, see: C. M. Casado, I. Cuadrado, M. Morán, B. Alonso, B. Garcia, B. Gonzales, J. Losada, Coord. Chem. Rev. 1999, 185–186, 53–79; S. H. Hwang, C. D. Shreiner, C. N. Moorefield, G. R. Newkome, New J. Chem. 2007, 31, 1027–1038; D. Astruc, C. Ornelas, J. Ruiz, Acc. Chem. Res. 2008, 41, 841–856.
- 2P. Nguyen, P. Gomez-Elipe, I. Manners, Chem. Rev. 1999, 99, 1515–1548; J. C. Eloi, L. Chabanne, G. R. Whittell, I. Manners, Mater. Today 2008, 11, 28–36. For the electrochemistry of multi-Fc polymers, see: R. Rulkens, A.-J. Lough, I. Manners, S. R. Lovelace, C. Grant, W. E. Geiger, J. Am. Chem. Soc. 1996, 118, 12683–12695.
- 3J. Losada, M. P. G. Armada, M. Zamora, B. Alonso, I. Cuadrado, C. M. Casado, Bioelectrochemistry 2006, 69, 65; J. Losada, M. Zamora, P. G. Armada, I. Cuadrado, B. Alonso, C. M. Casado, Anal. Bioanal. Chem. 2006, 385, 1209; F. J. Martinez, B. Gonzalez, B. Alonso, J. Losada, M. P. Garcia-Armada, C. M. Casado, J. Inorg. Organomet. Polym. Mater. 2008, 18, 51.
- 4M.-C. Daniel, J. Ruiz, D. Astruc, J. Am. Chem. Soc. 2003, 125, 1150–1151; C. Ornelas, J. Ruiz, C. Belin, D. Astruc, J. Am. Chem. Soc. 2009, 131, 590–601.
- 5For reviews on redox-active metallodendrimers, see: G. R. Newkome, E. He, C. N. Moorefield, Chem. Rev. 1999, 99, 1689–1746; M. Venturi, S. Serroni, A. Juris, S. Campagna, V. Balzani in Dendrimers, Topics Curr. Chem., Vol. 197 (Ed.: ), Springer, Heldelberg, 1998, pp. 193–228; C. M. Casado, M. Morán, B. Alonso, J. Losada, Coord. Chem. Rev. 1999, 193–195, 395–445; D. Astruc, E. Boisselier, C. Ornelas, Chem. Rev. 2010, 110, 1857–1959.
- 6T. Horikoshi, M. Itoh, M. Kurihara, K. Kubo, H. Nishihara, J. Electroanal. Chem. 1999, 473, 113–116; H. Nishihara, Bull. Soc. Chem. Jpn. 2001, 74, 19–29; M. Yamada, H. Nishihara, Chem. Commun. 2002, 2578–2579; M. Yamada, H. Nishihara, Eur. Phys. J. 2003, 24, 257–260; M. Yamada, H. Nishihara, Langmuir 2003, 19, 8050–8056; M. Yamada, H. Nishihara, ChemPhysChem 2004, 5, 555–559; M. Yamada, T. Tadera, K. Kubo, H. Nishihara, J. Phys. Chem. B 2003, 107, 3703–3711; M. Muraa, H. Nishihara, J. Inorg. Organomet. Polym. 2005, 15, 147–156.
- 7C. A. Nijhuis, K. A. Dolatowska, B. J. Ravoo, J. Huskens, D. N. Reinhoudt, Chem. Eur. J. 2007, 13, 69–80.
- 8Y. Ochi, M. Suzuki, T. Imaoka, M. Murata, H. Nishihara, Y. Einaga, K. Yamamoto, J. Am. Chem. Soc. 2010, 132, 5061–5069.
- 9D. O. Cowan, F. Kaufman, J. Am. Chem. Soc. 1970, 92, 219–220; D. O. Cowan, F. Kaufman, J. Am. Chem. Soc. 1971, 93, 3889–3893; C. Levanda, D. O. Cowan, K. Bechgaard, J. Am. Chem. Soc. 1975, 97, 1980–1981; M. J. Powers, T. J. Meyer, J. Am. Chem. Soc. 1978, 100, 4393–4398.
- 10M. Yamada, Bull. Soc. Chim. Jpn. 2009, 82, 152–170.
- 11R. F. Kovar, M. D. Rausch, J. Org. Chem. 1972, 35, 351–366.
- 12G. Doisneau, G. Balavoine, T. Fillebeen-Khan, J. Organomet. Chem. 1992, 425, 113–117.
- 13V. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708;
10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2596;10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google ScholarC. W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057–3064; M. Meldal, C. W. Tornøe, Chem. Rev. 2008, 108, 2952–3015. For a review of “click” dendrimers, see: G. Franc, A. Kakkar, Chem. Commun. 2008, 5267–5276.
- 14G. R. Newkome, Z. Yao, G. R. Baker, V. K. Gupta, J. Org. Chem. 1985, 50, 2003–2004; G. R. Newkome, Pure Appl. Chem. 1998, 70, 2337–2343; V. V. Narayanan, G. R. Newkome, Top. Curr. Chem. 1998, 197, 19–33.
- 15
- 15aGeneration 0 of azido dendrimers resulted from π-C5H5Fe-induced nona-allylation of mesitylene including removal of the organoiron group using visible-light photolysis,[15b] and subsequent hydrosilylation with HSi(CH2Cl)Me2 and azidation using NaN3.[16]
- 15bF. Moulines, L. Djakovitch, R. Boese, B. Gloaguen, W. Thiel, J.-L. Fillaut, M.-H. Delville, D. Astruc, Angew. Chem. 1993, 105, 1132–1134; Angew. Chem. Int. Ed. Engl. 1993, 32, 1075–1077; D. Catheline, D. Astruc, J. Organomet. Chem. 1983, 248, c 9–c12; V. Sartor, L. Djakovitch, J.-L. Fillaut, F. Moulines, F. Neveu, V. Marvaud, J. Guittard, J.-C. Blais, D. Astruc, J. Am. Chem. Soc. 1999, 121, 2929–2930.
- 16
- 16aSubsequent generations were constructed with a known iteration consisting of a Williamson reaction of the iodomethylsilyl-terminated dendrimers with the phenol triallyl dendron p-HO-C6H4C(CH2CHCH2)3, followed by the same hydrosilylation and azidation reactions.[16b]
- 16bJ. Ruiz, G. Lafuente, S. Marcen, C. Ornelas, S. Lazare, J.-C. Blais, E. Cloutet, D. Astruc, J. Am. Chem. Soc. 2003, 125, 7250–7257;
C. Ornelas, J. Ruiz, E. Cloutet, S. Alves, D. Astruc, Angew. Chem. 2007, 119, 890–895;
10.1002/ange.200602858 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 872–877; D. Astruc, J. Ruiz, Tetrahedron 2010, 66, 1769–1785.
- 17P. G. de Gennes, H. Hervet, J. Phys. Lett. 1983, 44, 351–360; K. Naidoo, S. J. Hugues, J. R. Moss, Macromolecules 1999, 32, 331; D. Pötschke, M. Ballauf, P. Lindner, M. Fischer, F. Vögtle, Macromolecules 1999, 32, 4079-.
- 18
- 18aFor a review of the distinct factors involving redox recognition of oxoanions by polyFc hosts, see: P. D. Beer, Chem. Commun. 1996, 689–696;
P. D. Beer, Acc. Chem. Res. 1998, 31, 71–80;
P. D. Beer, P. A. Gale, Angew. Chem. 2001, 113, 502–532;
10.1002/1521-3757(20010202)113:3<502::AID-ANGE502>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 2001, 40, 486–516;10.1002/1521-3773(20010202)40:3<486::AID-ANIE486>3.0.CO;2-P CAS PubMed Web of Science® Google ScholarM. C. Daniel, J. Ruiz, J.-C. Blais, N. Daro, D. Astruc, Chem. Eur. J. 2003, 9, 4371–4379; D. Astruc, M.-C. Daniel, J. Ruiz, Chem. Commun. 2004, 2637–2649;
- 18bfor ATP sensing by Fc sensors, see: O. Reynes, J. C. Moutet, J. Pecaut, G. Royal, E. Saint-Aman, New J. Chem. 2002, 26, 9–12;
- 18cfor ion-pairing interactions, see: A. Loupy, B. Tchoubar, D. Astruc, Chem. Rev. 1992, 92, 1141–1165; for dendritic ion-pairing effects, see: C. Ornelas, J. Ruiz, D. Astruc, Organometallics 2009, 28, 4431–4437.
- 19aN. Camine, U.-T. Mueller-Westerhoff, W. E. Geiger, J. Organomet. Chem. 2001, 637–639, 823–826; F. Barrière, N. Camine, W. E. Geiger, J. Am. Chem. Soc. 2002, 124, 7262–7263;
- 19bF. Barrière, W. E. Geiger, J. Am. Chem. Soc. 2006, 128, 3980–3989;
- 19cF. Barrière, W. E. Geiger, Acc. Chem. Res. 2010, 43, 1030–1039.
- 19dThe E2−E1 value is found to be only slightly larger for these monomeric biferrocenes (by 35 mV) than for the dendrimers with [nBu4N][PF6], but more significantly larger for the monomers than for the dendrimers (by 120 mV) with [nBu4N][B{meta-C6H3(CF3)2}4].
- 20S. Badèche, J.-C. Daran, J. Ruiz, D. Astruc, Inorg. Chem. 2008, 47, 4903–4908.
- 21
- 21aJ. G. de Vries, Dalton Trans. 2006, 421–429;
- 21bA. K. Diallo, C. Ornelas, L. Salmon, J. Ruiz, D. Astruc, Angew. Chem. 2007, 119, 8798–8802;
10.1002/ange.200703067 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8644–8648;
- 21cC. Ornelas, J. Ruiz, L. Salmon, D. Astruc, Adv. Synth. Catal. 2008, 350, 837–845.
- 22M. B. Robin, B. Melvin, P. Day, Adv. Inorg. Chem. Radiochem. 1968, 10, 247–422.