Factors Determining the Selection of Organic Reactions by Medicinal Chemists and the Use of These Reactions in Arrays (Small Focused Libraries)
Tony W. J. Cooper
Respiratory CEDD, GlaxoSmithKline, Gunnels Wood Road, Stevenage, SG1 2NY (UK), Fax: (+44) 1438-762-302
Search for more papers by this authorIan B. Campbell
Respiratory CEDD, GlaxoSmithKline, Gunnels Wood Road, Stevenage, SG1 2NY (UK), Fax: (+44) 1438-762-302
Search for more papers by this authorDr. Simon J. F. Macdonald
Respiratory CEDD, GlaxoSmithKline, Gunnels Wood Road, Stevenage, SG1 2NY (UK), Fax: (+44) 1438-762-302
Search for more papers by this authorTony W. J. Cooper
Respiratory CEDD, GlaxoSmithKline, Gunnels Wood Road, Stevenage, SG1 2NY (UK), Fax: (+44) 1438-762-302
Search for more papers by this authorIan B. Campbell
Respiratory CEDD, GlaxoSmithKline, Gunnels Wood Road, Stevenage, SG1 2NY (UK), Fax: (+44) 1438-762-302
Search for more papers by this authorDr. Simon J. F. Macdonald
Respiratory CEDD, GlaxoSmithKline, Gunnels Wood Road, Stevenage, SG1 2NY (UK), Fax: (+44) 1438-762-302
Search for more papers by this authorGraphical Abstract
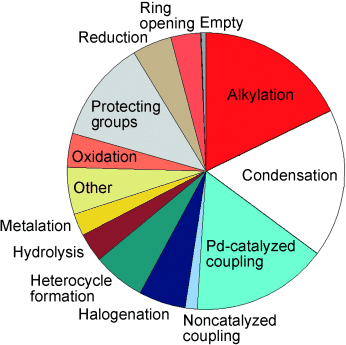
Helping chemists help chemists: What reactions do medicinal chemists use in drug discovery? (The pie chart shows a snapshot from one unit at GlaxoSmith-Kline.) What criteria do they use to select synthetic methodology? Why are arrays (small focused libraries) so powerful in the lead-optimization process? These questions are considered in this Minireview, which also describes attempts to expand the number of robust reactions available to medicinal chemists.
Abstract
Synthetic organic reactions are a fundamental enabler of small-molecule drug discovery, and the vast majority of medicinal chemists are initially trained—either at universities or within industry—as synthetic organic chemists. The sheer breadth of synthetic methodology available to the medicinal chemist represents an almost endless source of innovation. But what reactions do medicinal chemists use in drug discovery? And what criteria do they use in selecting synthetic methodology? Why are arrays (small focused libraries) so powerful in the lead-optimization process? In this Minireview, we suggest some answers to these questions and also describe how we have tried to expand the number of robust reactions available to the medicinal chemist.
References
- 1
- 1aS. T. Paul, D. S. Mytelka, C. T. Dunwiddie, C. C. Persinger, B. H. Munos, S. R. Lindborg, A. L. Schacht, Nat. Rev. Drug Discovery 2010, 9, 203–214;
- 1bI. Kola, J. Landis, Nat. Rev. Drug Discovery 2004, 3, 711–715;
- 1cH. Kubiyi, Nat. Rev. Drug Discovery 2003, 2, 665–668;
- 1dan analysis reported in a KMR Pharmaceutical Benchmarking Forum R&D General Metrics Study Presentation on May 4, 2006 stated that the overall new-medical-entity-attrition median (i.e. the median percentage of drug candidates that didn′t make it to market) for 2002–2006 in the pharmaceutical industry was 91 %.
- 2J. A. Kramer, J. E. Sagartz, D. L. Morris, Nat. Rev. Drug Discovery 2007, 6, 636–649.
- 3P. D. Leeson, B. Springthorpe, Nat. Rev. Drug Discovery 2007, 6, 881–890.
- 4Log P is the partition coefficient of a compound between octanol and water. Lipophilicity can be calculated and described as clog P (with varying degrees of accuracy), which is a very widely used parameter in medicinal chemistry. ACD labs offer a free clog P calculator http://www.acdlabs.com/download/logp.html. In a seminal study, Lipinski et al. investigated the lipophilicity of oral drugs (among other compounds) and suggested that for good absorption and permeability (important factors in the discovery of oral drugs) the clogP value should be less than 5 ( C. A. Lipinski, F. Lombardo, B. W. Dominy, P. J. Feeney, Adv. Drug Delivery Rev. 1997, 23, 3–25). In fact, this value is now viewed as being at the upper end of desirable lipophilicity for an oral drug; clinical compounds with a clogP value between 1 and 3 are preferred. Correlations between the high lipophilicity of clinical candidates and increased attrition have become a recurring theme. Leeson and Springthorpe[3] from AstraZeneca commented on drug promiscuity (the tendency of a compound to modulate biological processes other than the desired process): “The consequences …︁ of the marked increase in lipophilicity include a greater likelihood of lack of selectivity and attrition in drug development.” Researchers from Pfizer wrote of “an increased likelihood of toxic events [that] was found for less polar, more lipophilic compounds” ( J. D. Hughes, J. Blagg, D. A. Price, S. Bailey, G. A. DeCrescenzo, R. V. Devraj, E. Ellsworth, Y. M. Fobian, M. E. Gibbs, R. W. Gilles, N. Greene, E. Huang, T. Krieger-Burke, J. Loesel, T. Wager, L. Whiteley, Y. Zhang, Bioorg. Med. Chem. Lett. 2008, 18, 4872). Gleeson from GlaxoSmithKline noted “the need to focus on a lower molecular weight and logP area of physicochemical property space” ( M. P. Gleeson, J. Med. Chem. 2008, 51, 817–834). In relation to compound permeability (the ability of a compound to cross membranes): “logD and molecular weight are the most important factors” ( M. J. Waring, Bioorg. Med. Chem. Lett. 2009, 19, 2844–2851). We found that “adding more lipophilicity by adding more aromatic rings …︁ is likely to increase the risk of attrition” ( T. J. Ritchie, S. J. F. Macdonald, Drug Discovery Today 2009, 14, 1011–1020). Regarding compounds for lead optimization: “key properties [such as lipophilicity] of recently developed clinical candidates and advanced lead compounds have been shown to differ significantly from those of historical leads and drugs” ( G. M. Keserü, G. M. Makara, Nat. Rev. Drug Discovery 2009, 8, 203–212).
- 5For an analysis of reactions used in the large-scale preparation of drug-candidate molecules, see: J. S. Carey, D. Laffan, C. Thomson, M. T. Williams, Org. Biomol. Chem. 2006, 4, 2337–2347.
- 6Diversity-oriented synthesis (DOS; T. E. Nielsen, S. L. Schreiber, Angew. Chem. 2008, 120, 52–61;
10.1002/ange.200703073 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 48–56) provides valuable tools for chemical biology. However, many of the molecules generated are structurally complex and have relatively high molecular weights and lipophilicity. These properties hinder their use as potential leads for oral-drug-discovery programs. High structural complexity reduces hit rates and can make optimization chemistry difficult ( M. M. Hann, A. R. Leach, G. Harper, J. Chem. Inf. Comput. Sci. 2001, 41, 856–864).
- 7As a compound gets closer to becoming a clinical candidate, it becomes a “higher-value” compound, and more time is often invested in refining the synthetic route.
- 8Clearly, there are many exceptions to this statement. However, the introduction of a stereogenic center (or a further stereogenic center) in a lead series is rarely undertaken lightly, particularly when the stereogenic center is not embedded in a commercially available molecule or cannot be introduced with good stereoselectivity. “Development” refers to the activities between the discovery of the clinical candidate and its launch onto the market. It is a highly resource intensive and expensive process and includes scaling up, toxicity studies, clinical trials, and registering the drug with the regulatory authorities (e.g., FDA) regulatory. When a candidate has numerous isomers, there can be a significantly increased burden to show that the biological activity resides in a particular isomer and that the properties and quantities of the undesired isomers are both known and controlled.
- 9F. Lovering, J. Bikker, C. Humblet, J. Med. Chem. 2009, 52, 6752–6756. In questioning whether “more is lost …︁ than gained by avoiding cutting edge synthetic technologies …︁ [and not] taking a little more time to introduce chiral centers”, one of the referees raised a fair point. In our experience, considerable nerve is required from the medicinal chemist to justify the “time delays” that might result.
- 10Frequently, filters are used to flag or exclude particular types of structure or functionality in an attempt to decrease drug-discovery attrition: J. B. Baell, G. A. Holloway, J. Med. Chem. 2010, 49, 1433–1441 and references therein; see also: http://ncgc.nih.gov/projects/cruzain/Cruzain_qHTS_Supplemental_ Table_Exclusion_Filters.xls.
- 11S. J. F. Macdonald, P. W. Smith, Drug Discovery Today 2001, 6, 947–953.
- 12The EPSRC website is: www.epsrc.ac.uk.
- 13We have encouraged the PhD students and postdoctoral research assistants to apply their new methodology to the preparation of arrays with only partial success. However, when the new methodology has been applied to the preparation of arrays, it has proved a valuable learning experience for the students involved for many of the reasons outlined herein (especially in terms of working with more-polar molecules with more functional groups). The students begin to appreciate the multidisciplinary approach to drug discovery and that working in a team saves much time and effort in comparison to working by themselves. The experience of working in a team, the use of more automated equipment, and their introduction to the concept that in early lead optimization the synthesis of 2 mg of a compound for testing is more important than the overall yield provide the students with an industrial perspective. They also form valuable links with industrial colleagues; these connections should be useful to them in their future careers.
- 14For examples of the studies that have resulted from this initiative, see: “Palladium catalyzed tandem alkenyl- and aryl-CN bond formation: a cascade N-annulation route to 4-, 5-, 6- and 7-chloroindoles”, L. C. Henderson, M. J. Lindon, M. C. Willis, Tetrahedron, in press; “A direct route to triazole boronic esters and their application in the synthesis of small molecule arrays”, J. Huang, S. J. F. Macdonald, A. W. J. Cooper, G. Fisher, J. P. A. Harrity, Tetrahedron Lett. 2009, 50, 5539–5541; “The rhodium carbene route to oxazoles: a remarkable catalyst effect”, B. Shi, A. J. Blake, I. B. Campbell, B. D. Judkins, C. J. Moody, Chem. Commun. 2009, 3291–3293; “Convergent synthesis of dihydroquinolones from o-aminoarylboronates”, J. Horn, H. Y. Li, S. P. Marsden, A. Nelson, R. J. Shearer, A. J. Campbell, D. House, G. G. Weingarten, Tetrahedron 2009, 65, 9002–9007; “Convergent, Regiospecific Synthesis of Quinolines from o-Aminophenylboronates”, J. Horn, S. P. Marsden, A. Nelson, D. House, G. G. Weingarten, Org. Lett. 2008, 10, 4117–4120; “Analysis of Neighborhood Behavior in Lead Optimization and Array Design”, G. Papadatos, A. W. J. Cooper, V. Kadirkamanathan, S. J. F. Macdonald, I. M. McLay, S. D. Pickett, J. M. Pritchard, P. Willett, V. J. Gillet, J. Chem. Inf. Model. 2009, 49, 195–208; “Rearrangement Strategy for the Synthesis of 2-Aminoanilines”, A. Porzelle, M. D. Woodrow, N. C. O. Tomkinson, Org. Lett. 2010, 12, 1492–1495; “Synthesis of Benzoxazolones from Nitroarenes or Aryl Halides”, A. Porzelle, M. D. Woodrow, N. C. O. Tomkinson, Org. Lett. 2010, 12, 812–815; “Facile Procedure for the Synthesis of N-Aryl-N-hydroxy Carbamates”, A. Porzelle, M. D. Woodrow, N. C. O. Tomkinson, Synlett 2009, 798–802.
- 15The first report of new methodology carries considerable kudos and usually appears in high-impact journals. Frequently, however, it is sufficient to demonstrate that the methodology “works” in high yields on a limited range of substrates. Functionality which may interfere with the methodology can be omitted because the novelty factor of the methodology alone is generally sufficient for acceptance by referees. The subsequent demonstration of a refined protocol compatible with a wide range of functionality appears to be valued less, perhaps because it is less novel and less amenable to publication in high-impact journals. The pressure to produce high-impact publications may reduce the incentive for such studies to be carried out. Nonetheless, it is often a subsequent study which indicates the value of the new methodology to the medicinal chemist.
- 16The links to the NIH website describing this initiative and the centers are: www.nigms.nih.gov/Initiatives/CMLD; www.nigms. nih.gov/Initiatives/CMLD/Centers/.
- 17Frequently, the synthesis of many monomers and substrates is outsourced.
- 18Over this time period, the synthesis of very few arrays was outsourced. We have found it much quicker and logistically much easier for arrays to be made in-house.
- 19The medicinal-chemistry team charged with carrying out the lead-optimization process, which results in a clinical candidate, typically comprises (in decreasing order of seniority): a team leader (an experienced medicinal chemist and manager, usually with a PhD), two or three experienced medicinal chemists (graduates with or without a PhD), one or two less experienced graduates, and one or two university students on a one-year placement. All except the team leader carry out synthetic work; all except the students contribute towards the medicinal chemistry commensurate with their grade and experience.
- 20S. M. Free, J. W. Wilson, J. Med. Chem. 1964, 7, 395–399.
- 21K. McClure, M. Hack, L. Huang, C. Sehon, M. Morton, L. Li, T. D. Barrett, N. Shankley, J. G. Breitenbucher, Bioorg. Med. Chem. Lett. 2006, 16, 72–76.
- 22W. Zhai, N. Flynn, D. A. Longhi, J. A. Tino, B. J. Murphy, D. Slusarchyk, D. A. Gordon, A. Pendri, S. Shi, R. Stoffel, B. Ma, M. J. Sofia, S. W. Gerritz, Bioorg. Med. Chem. Lett. 2008, 18, 5083–5086.
- 23Historically, the majority of reactions were carried out on a less than 10 g scale owing to difficulties in purification; as a result, intermediate availability may have been limited. However (outside of cost constraints or limited commercial availability of the starting materials), intermediate availability is now less of an issue for two reasons: first, the advent of multiple vendors offering the cheap synthesis of bespoke intermediates, and second, the ready availability of purification systems which can readily handle 50–100 g of crude material.