Enantioselective Synthesis of Oasomycin A, Part III: Fragment Assembly and Confirmation of Structure†
David A. Evans Prof.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617–495–1460
Search for more papers by this authorPavel Nagorny
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617–495–1460
Search for more papers by this authorKenneth J. McRae Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617–495–1460
Search for more papers by this authorLouis-Sebastian Sonntag Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617–495–1460
Search for more papers by this authorDominic J. Reynolds Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617–495–1460
Search for more papers by this authorFilisaty Vounatsos Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617–495–1460
Search for more papers by this authorDavid A. Evans Prof.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617–495–1460
Search for more papers by this authorPavel Nagorny
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617–495–1460
Search for more papers by this authorKenneth J. McRae Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617–495–1460
Search for more papers by this authorLouis-Sebastian Sonntag Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617–495–1460
Search for more papers by this authorDominic J. Reynolds Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617–495–1460
Search for more papers by this authorFilisaty Vounatsos Dr.
Department of Chemistry & Chemical Biology, Harvard University, Cambridge, MA 02138, USA, Fax: (+1) 617–495–1460
Search for more papers by this authorFinancial support has been provided by the National Institutes of Health (GM-33327-19), the Merck Research Laboratories, Amgen, and Eli Lilly. A postdoctoral fellowship was provided to L.-S.S. by the Deutscher Akademischer Austauschdienst and the Novartis Foundation, and to D.J.R. by the Glaxo Foundation.
Graphical Abstract
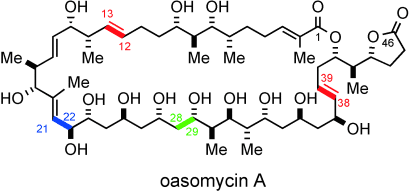
Putting the pieces together: The total synthesis of the natural macrolide oasomycin A has been realized. Key fragment couplings include an anti-Felkin selective aldol addition (green), Kociensky–Julia olefinations (red), and competitive Weinreb amide acylation reaction (blue). The utility of the 4,5-diphenyloxazole as a carboxy surrogate and the late-stage macrolactonization affording the 42-membered macrocycle of oasomycin A are also described.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z603652_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. A. Evans, P. Nagorny, K. J. McRae, D. J. Reynolds, L.-S. Sonntag, F. Vounatsos, R. Xu, Angew. Chem. 2007, 119, 543–546; Angew. Chem. Int. Ed. 2007, 46, 537–540;
- 1bD. A. Evans, P. Nagorny, D. J. Reynolds, K. J. McRae, Angew. Chem. 2007, 119, 547–550; Angew. Chem. Int. Ed. 2007, 46, 541–544.
- 2M. Mayer, R. Thiericke, J. Chem. Soc. Perkin Trans. 1 1993, 2525–2531.
- 3P. J. Kocienski, A. Bell, P. R. Blakemore, Synlett 2000, 365–366. For a recent review on this topic, see: P. R. Blakemore, J. Chem. Soc. Perkin Trans. 1 2002, 2563–2585.
- 4Our attempts to further optimize the Kocienski–Julia olefination were unsuccessful. In our model studies, the use of LiHMDS eliminates the side product, but decreases the E/Z ratio of the Δ12-olefin isomers to 2:1. Protection of the C15 alcohol as a triisopropylsilyl ether significantly diminishes the reactivity of the aldehyde because of steric hindrance.
- 5
- 5aI. Paterson, K. R. Gibson, R. M. Oballa, Tetrahedron Lett. 1996, 37, 8585–8588;
- 5bD. A. Evans, P. J. Coleman, B. Côté, J. Org. Chem. 1997, 62, 788–789.
- 6
- 6aD. A. Evans, M. J. Dart, J. L. Duffy, J. Am. Chem. Soc. 1996, 118, 4322–4343;
- 6bD. A. Evans, J. L. Duffy, M. J. Dart, Tetrahedron Lett. 1994, 35, 8537–8540.
- 7
- 7aD. A. Evans, B. Côté, P. J. Coleman, B. T. Connell, J. Am. Chem. Soc. 2003, 125, 10893–10898;
- 7bL. C. Dias, A. M. Aguilar, A. G. Salles, L. J. Steil, W. R. Roush, J. Org. Chem. 2005, 70, 10461–10465.
- 8D. A. Evans, P. Nagorny, unpublished results. Also see Ref. [7a] for precedent for this coupling.
- 9The stereochemistry of the C29 stereocenter was proven by the Mosher ester analysis. See: J. A. Dale, H. S. Mosher, J. Am. Chem. Soc. 1973, 95, 512–519.
- 10E. Kim, D. M. Gordon, W. Schmid, G. M. Whitesides, J. Org. Chem. 1993, 58, 5500–5507.
- 11The inseparable impurity (ca. 9 %) that arose from the Wacker oxidation of 3 a and that was present in the ketone 8 starting material obscured the precise determination of the reaction diastereoselectivity with the lower limit of detection set at 10:1.
- 12The stereochemistry of the reduction was proven by analysis of the 13C NMR shift of the acetonide moiety. See:
- 12aS. D. Rychnovsky, D. J. Skalitzky, Tetrahedron Lett. 1990, 31, 945–948;
- 12bS. D. Rychnovsky, B. Rogers, G. Yang, Tetrahedron Lett. 1990, 31, 3511–3515;
- 12cD. A. Evans, D. L. Rieger, J. R. Gage, Tetrahedron Lett. 1990, 31, 7099–7100.
- 13J. Inanaga, K. Hirata, H. Saeki, T. Katsuki, M. Yamaguchi, Bull. Chem. Soc. Jpn. 1979, 52, 1989–1993.
- 14
- 14aM. Hikota, H. Tone, K. Horita, O. Yonemitsu, J. Org. Chem. 1990, 55, 7–9;
- 14bI. Shiina, M. Kubota, R. Ibuka, Tetrahedron Lett. 2002, 43, 7535–7539;
- 14cE. P. Boden, G. E. Keck, J. Org. Chem. 1985, 50, 2394–2395.
- 15For further details, see the Supporting Information.
- 16We found the optimal concentration of HF for the deprotection was 1–2 M, at 7 °C as more concentrated solutions of HF or higher temperatures decompose oasomycin A. Minor amounts of mono-TBS-protected oasomycin A (ca. 10–20 %) were also recovered after workup and then recycled. The yield reported was calculated after one such recycling.
- 17We thank Professor Y. Kishi for providing the natural samples of oasomycin A and B.
- 18S. Grabley, G. Kretzschmar, M. Mayer, S. Philipps, R. Thiericke, J. Wink, A. Zeeck, Liebigs Ann. Chem. 1993, 5, 573–579.
10.1002/jlac.199319930193 Google Scholar
- 19Y. Kobayashi, S.-H. Tan, Y. Kishi, J. Am. Chem. Soc. 2001, 123, 2076–2078, and references therein.
- 20A. Parenty, X. Moreau, J. -M. Campagne, Chem. Rev. 2006, 106, 911–939.
- 21I. Paterson, K.-S. Yeung, R. A. Ward, J. D. Smith, J. G. Cumming, S. Lamholey, Tetrahedron 1995, 51, 9467–9486.