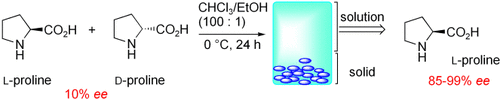
Large Nonlinear Effect Observed in the Enantiomeric Excess of Proline in Solution and That in the Solid State†
Yujiro Hayashi Prof. Dr.
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorMasayoshi Matsuzawa
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorJunichiro Yamaguchi
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorSayaka Yonehara
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorYasunobu Matsumoto
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorMitsuru Shoji Dr.
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorDaisuke Hashizume Dr.
RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan
Search for more papers by this authorHiroyuki Koshino Dr.
RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan
Search for more papers by this authorYujiro Hayashi Prof. Dr.
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorMasayoshi Matsuzawa
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorJunichiro Yamaguchi
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorSayaka Yonehara
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorYasunobu Matsumoto
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorMitsuru Shoji Dr.
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorDaisuke Hashizume Dr.
RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan
Search for more papers by this authorHiroyuki Koshino Dr.
RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan
Search for more papers by this authorThis work was partially supported by Toray Science Foundation and a Grant-in-Aid for Scientific Research on Priority Areas 16073219 from MEXT. We thank Professors K. Soai (Tokyo University of Science) and K. Saigo (The University of Tokyo) for helpful discussions.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2006/z601506_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Mason, Chem. Soc. Rev. 1988, 17, 347;
- 1bW. A. Bonner, Origins Life Evol. Biosphere 1991, 21, 59;
- 1cB. L. Feringa, R. A. van Delden, Angew. Chem. 1999, 111, 3624;
10.1002/(SICI)1521-3757(19991203)111:23<3624::AID-ANGE3624>3.0.CO;2-X Web of Science® Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3418;10.1002/(SICI)1521-3773(19991203)38:23<3418::AID-ANIE3418>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 1dM. Avalos, R. Babiano, P. Cintas, J. L. Jimenez, J. C. Palacios, Chem. Commun. 2000, 887;
- 1eH. Buschmann, R. Thede, D. Heller, Angew. Chem. 2000, 112, 4197;
10.1002/1521-3757(20001117)112:22<4197::AID-ANGE4197>3.0.CO;2-5 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 4033;10.1002/1521-3773(20001117)39:22<4033::AID-ANIE4033>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar
- 1fJ. Podlech, Cell. Mol. Life Sci. 2001, 58, 44.
- 2
- 2aK. Soai, T. Shibata, H. Morioka, K. Choji, Nature 1995, 378, 767;
- 2bK. Soai, T. Shibata, I. Sato, Acc. Chem. Res. 2000, 33, 382;
- 2cK. Soai, I. Sato, T. Shibata, Chem. Rec. 2001, 1, 321;
- 2dK. Soai, T. Shibata, I. Sato, Bull. Chem. Soc. Jpn. 2004, 77, 1063.
- 3S. P. Matthew, H. Iwamura, D. G. Blackmond, Angew. Chem. 2004, 116, 3379;
10.1002/ange.200453997 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3317.
- 4
- 4aS. Pizzarello, A. L. Weber, Science 2004, 303, 1151;
- 4bA. B. Northrup, D. W. C. MacMillan, Science 2004, 305, 1752;
- 4cD. Enders, C. Grondal, Angew. Chem. 2005, 117, 1235;
10.1002/ange.200462428 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1210;
- 4dJ. T. Suri, D. B. Ramachary, C. F. Barbas III, Org. Lett. 2005, 7, 1383;
- 4eA. Cordova, I. Ibrahem, J. Casas, H. Sunden, M. Engqvist, E. Reyes, Chem. Eur. J. 2005, 11, 4772;
- 4fD. Enders, J. Palecek, C. Grondal, Chem. Commun. 2006, 655;
- 4gC. Grondal, D. Enders, Tetrahedron 2006, 62, 329;
- 4hI. Ibrahem, A. Cordova, Tetrahedron Lett. 2005, 46, 3363;
- 4iN. S. Chowdari, D. B. Ramachary, A. Cordova, C. F. Barbas III, Tetrahedron Lett. 2002, 43, 9591;
- 4jfor a review of proline-catalyzed synthesis of carbohydrates, see: D. Enders, M. Voith, A. Lenzen, Angew. Chem. 2005, 117, 1330;
10.1002/ange.200400659 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1304.
- 5A. Cordova, M. Engqvist, I. Ibrahem, J. Casas, H. Sunden, Chem. Commun. 2005, 2047.
- 6Reviews:
- 6aP. I. Dalko, L. Moisan, Angew. Chem. 2001, 113, 3840;
10.1002/1521-3757(20011015)113:20<3840::AID-ANGE3840>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2001, 40, 3726;10.1002/1521-3773(20011015)40:20<3726::AID-ANIE3726>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 6bM. Movassaghi, E. N. Jacobsen, Science 2002, 298, 1904;
- 6cB. List, Acc. Chem. Res. 2004, 37, 548;
- 6dP. I. Dalko, L. Moisan, Angew. Chem. 2004, 116, 5248;
10.1002/ange.200400650 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5138;
- 6eA. Berkessel, H. Groger, Asymmetric Organocatalysis, Wiley-VCH, Weinheim, 2005;
- 6ffor reviews on the utilization of amino acids in stereoselective synthesis, see: K. Drauz, A. Kleeman, J. Martens, Angew. Chem. 1982, 94, 590; Angew. Chem. Int. Ed. Engl. 1982, 21, 584;
- 6gJ. Martens, Top. Curr. Chem. 1984, 125, 165.
- 7
- 7aZ. G. Hajos, D. R. Parrish, German Patent DE 2102623, 1971;
- 7bU. Eder, G. Sauer, R. Wiechert, Angew. Chem. 1971, 83, 492;
10.1002/ange.19710831307 Google ScholarAngew. Chem. Int. Ed. Engl. 1971, 10, 496.
- 8B. List, R. A. Lerner, C. F. Barbas III, J. Am. Chem. Soc. 2000, 122, 2395.
- 9For selected studies of proline-catalyzed aldol reactions, see:
- 9aW. Notz, B. List, J. Am. Chem. Soc. 2000, 122, 7386;
- 9bK. Sakthivel, W. Notz, T. Bui, C. F. Barbas III, J. Am. Chem. Soc. 2001, 123, 5260;
- 9cA. B. Northrup, D. W. C. MacMillan, J. Am. Chem. Soc. 2002, 124, 6798;
- 9dfor a review, see: B. List in Modern Aldol Reactions, Vol. 1 (Ed.: ), Wiley-VCH, Weinheim, 2004, p. 161.
- 10A. Cordova, Acc. Chem. Res. 2004, 37, 102.
- 11R. O. Duthaler, Angew. Chem. 2003, 115, 1005;
10.1002/ange.200390228 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 975.
- 12
- 12aG. Zhong, Angew. Chem. 2003, 115, 4379; Angew. Chem. Int. Ed. 2003, 42, 4247;
- 12bS. P. Brown, M. P. Brochu, C. J. Sinz, D. W. C. MacMillan, J. Am. Chem. Soc. 2003, 125, 10808;
- 12cY. Hayashi, J. Yamaguchi, K. Hibino, M. Shoji, Tetrahedron Lett. 2003, 44, 8293;
- 12dA. Bøgevig, H. Sunden, A. Cordova, Angew. Chem. 2004, 116, 1129;
10.1002/ange.200353018 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1109;
- 12eY. Hayashi, J. Yamaguchi, T. Sumiya, M. Shoji, Angew. Chem. 2004, 116, 1132; Angew. Chem. Int. Ed. 2004, 43, 1112;
- 12fA. Cordova, H. Sunden, A. Bøgevig, M. Johansson, F. Himo, Chem. Eur. J. 2004, 10, 3673;
- 12gY. Hayashi, J. Yamaguchi, T. Sumiya, K. Hibino, M. Shoji, J. Org. Chem. 2004, 69, 5966;
- 12hfor a review, see: P. Merino, T. Tejero, Angew. Chem. 2004, 116, 3055; Angew. Chem. Int. Ed. 2004, 43, 2995.
- 13R. L. Kayushina, B. K. Vainshtein, Kristallografiya 1965, 10, 834.
- 14A CIF file for dl-proline was deposited with the Cambridge Crystallographic Data Centre. CCDC 254728 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15D. D. Perrin, W. L. F. Armarego, Purification of Laboratory Chemicals, Pergamon Press, Oxford, England, 1988.
- 16J. Jacques, A. Collet, S. H. Wilen, Enantiomers, Racemates and Resolutions, Wiley, New York, 1981.
- 17R. Tamura, D. Fujimoto, Z. Lepp, K. Misaki, H. Miura, H. Takahashi, T. Ushio, T. Nakai, K. Hirotsu, J. Am. Chem. Soc. 2002, 124, 13139.
- 18When N,N-dimethylformamide was used as a solvent in α-aminoxylation, a linear effect was observed.
- 19Blackmond and co-workers proposed an increase in solubility of proline caused by product, owing to a hydrogen-bonding interaction, see: H. Iwamura, D. H. Wells, Jr.,S. P. Mathew, M. Klussmann, A. Armstrong, D. G. Blackmond, J. Am. Chem. Soc. 2004, 126, 16312.
- 20A. Cordova, H. Sunden, M. Engqvist, I. Ibrahem, J. Casas, J. Am. Chem. Soc. 2004, 126, 8914.
- 21S. Pizzarello, M. Zolensky, K. A. Turk, Geochim. Cosmochim. Acta 2003, 67, 1589.
- 22G. M. M. Caro, U. J. Meierhenrich, W. A. Schutte, B. Barbier, A. A. Segovia, H. Rosenbauer, W. H.-P. Thiemann, A. Brack, J. M. Greenberg, Nature 2002, 416, 403.
- 23Note added in proof (June 12, 2006): Since this manuscript was accepted for publication, a paper by Blackmond and co-workers has appeared that describes a similar phenomenon (M. Klussmann, H. Iwamura, S. P. Mathew, D. H. Wells, Jr.,U. Pandya, A. Armstrong, D. G. Blackmond, Nature 2006, 441, 621). The results in our present Communication explain the greater stability of crystals of dl-proline over those of the separate enantiomers and provide a rationale for the nonlinear effects observed in the ee of proline in solution and hence in the aldol reaction.