Exceptionally Strong Electronic Communication through Hydrogen Bonds in Porphyrin–C60 Pairs†
Luis Sánchez Dr.
Departamento de Química Orgánica, Facultad de Química, Universidad Complutense de Madrid, 28040 Madrid, Spain, Fax: (+34) 91-394-4103
Search for more papers by this authorMaria Sierra
Departamento de Química Orgánica, Facultad de Química, Universidad Complutense de Madrid, 28040 Madrid, Spain, Fax: (+34) 91-394-4103
Search for more papers by this authorNazario Martín Prof. Dr.
Departamento de Química Orgánica, Facultad de Química, Universidad Complutense de Madrid, 28040 Madrid, Spain, Fax: (+34) 91-394-4103
Search for more papers by this authorAndrew J. Myles Dr.
The Skaggs Institute for Chemical Biology and Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road (MB26), La Jolla, CA 92037, USA, Fax: (+34) 91-394-4103
Search for more papers by this authorTrevor J. Dale
The Skaggs Institute for Chemical Biology and Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road (MB26), La Jolla, CA 92037, USA, Fax: (+34) 91-394-4103
Search for more papers by this authorJulius Rebek Jr. Prof. Dr.
The Skaggs Institute for Chemical Biology and Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road (MB26), La Jolla, CA 92037, USA, Fax: (+34) 91-394-4103
Search for more papers by this authorWolfgang Seitz
Institut für Physikalische und Theoretische Chemie, Universität Erlangen-Nürnberg, Egerlandstrasse 3, 91058 Erlangen, Germany, Fax: (+49) 9131-852-830
Search for more papers by this authorDirk M. Guldi Prof. Dr.
Institut für Physikalische und Theoretische Chemie, Universität Erlangen-Nürnberg, Egerlandstrasse 3, 91058 Erlangen, Germany, Fax: (+49) 9131-852-830
Search for more papers by this authorLuis Sánchez Dr.
Departamento de Química Orgánica, Facultad de Química, Universidad Complutense de Madrid, 28040 Madrid, Spain, Fax: (+34) 91-394-4103
Search for more papers by this authorMaria Sierra
Departamento de Química Orgánica, Facultad de Química, Universidad Complutense de Madrid, 28040 Madrid, Spain, Fax: (+34) 91-394-4103
Search for more papers by this authorNazario Martín Prof. Dr.
Departamento de Química Orgánica, Facultad de Química, Universidad Complutense de Madrid, 28040 Madrid, Spain, Fax: (+34) 91-394-4103
Search for more papers by this authorAndrew J. Myles Dr.
The Skaggs Institute for Chemical Biology and Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road (MB26), La Jolla, CA 92037, USA, Fax: (+34) 91-394-4103
Search for more papers by this authorTrevor J. Dale
The Skaggs Institute for Chemical Biology and Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road (MB26), La Jolla, CA 92037, USA, Fax: (+34) 91-394-4103
Search for more papers by this authorJulius Rebek Jr. Prof. Dr.
The Skaggs Institute for Chemical Biology and Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road (MB26), La Jolla, CA 92037, USA, Fax: (+34) 91-394-4103
Search for more papers by this authorWolfgang Seitz
Institut für Physikalische und Theoretische Chemie, Universität Erlangen-Nürnberg, Egerlandstrasse 3, 91058 Erlangen, Germany, Fax: (+49) 9131-852-830
Search for more papers by this authorDirk M. Guldi Prof. Dr.
Institut für Physikalische und Theoretische Chemie, Universität Erlangen-Nürnberg, Egerlandstrasse 3, 91058 Erlangen, Germany, Fax: (+49) 9131-852-830
Search for more papers by this authorThis work was supported by the MEC of Spain and Comunidad de Madrid (CTQ2005-02609/BTQ and P-PPQ-000225-0505), the Deutsche Forschungsgemeinschaft (SFB 583), FCI, the Office of Basic Energy Sciences of the US Department of Energy (contribution No. NDRL-4670 from the Notre Dame Radiation Laboratory), and the Skaggs Institute for Chemical Biology. M.S. is indebted to MEC of Spain for a PhD fellowship, A.J.M. is a NSERC postdoctoral fellow, and T.J.D. is a Skaggs predoctoral fellow.
Graphical Abstract
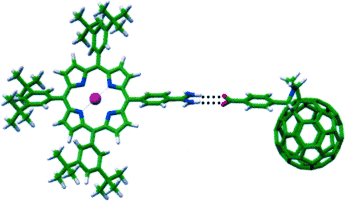
Hooking up: Self-assembly of a porphyrin amidine and fulleropyrrolidine carboxylic acid based on a two-point amidinium–carboxylate motif leads to supramolecular dyads of high stability (Ka≈107 M−1 in toluene/acetonitrile (9:1)). The synergy of the hydrogen bonds and electrostatic interactions has been shown to be particularly beneficial in terms of electronic coupling between both electroactive components of the dyads.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2006/z601264_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. A. Stubbe, D. G. Nocera, C. S. Yee, M. C. Y. Chang, Chem. Rev. 2003, 103, 2167–2201;
- 1bA. Namslauer, A. Aagaard, A. Katsonouri, P. Brzezinski, Biochemistry 2003, 42, 1488–1498.
- 2
- 2aA. P. H. J. Schenning, J. V. Herrikhuyzen, P. Jonkheijm, Z. Chen, F. Würthner, E. W. Meijer, J. Am. Chem. Soc. 2002, 124, 10252–10253;
- 2bJ. L. Sessler, M. Sathiosatham, C. T. Brown, T. A. Rhodes, G. Wiederrecht, J. Am. Chem. Soc. 2001, 123, 3655–3660;
- 2cA. J. Myles, N. R. Branda, J. Am. Chem. Soc. 2001, 123, 177–178;
- 2dR. K. Castellano, S. L. Craig, C. Nuckolls, J. Rebek, Jr., J. Am. Chem. Soc. 2000, 122, 7876–7882;
- 2eT. H. Ghaddar, E. W. Castner, S. S. Isied, J. Am. Chem. Soc. 2000, 122, 1233–1234.
- 3
- 3aJ. L. Sessler, B. Wang, A. Harriman, J. Am. Chem. Soc. 1993, 115, 10418–10419;
- 3bP. J. F. De Rege, S. A. Williams, M. J. Therien, Science 1995, 269, 1409–1413.
- 4
- 4aW. Ma, C. Yang, X. Gong, K. Lee, A. J. Heeger, Adv. Funct. Mater. 2005, 15, 1617–1622;
- 4bG. Li, V. Shrotriya, J. Huang, Y. Yao, T. Moriarty, K. Emery, Y. Yang, Nat. Mater. 2005, 4, 864–868;
- 4cI. Riedel, E. von Hauff, J. Parisi, N. Martín, F. Giacalone, V. Dyakonov, Adv. Funct. Mater. 2005, 15, 1979–1987;
- 4dM. W. Wienk, J. M. Kroon, W. J. H. Verhees, J. Knol, J. C. Hummelen, P. A. van Hal, R. A. J. Janssen, Angew. Chem. 2003, 115, 3493–3497;
10.1002/ange.200351647 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3371–3375;
- 4eC. J. Brabec, N. S. Sariciftci, J. C. Hummelen, Adv. Funct. Mater. 2001, 11, 15–26.
- 5
- 5aC.-H. Huang, N. D. McClenaghan, A. Kuhn, J. W. Hofstraat, D. M. Bassani, Org. Lett. 2005, 7, 3409–3412;
- 5bJ. L. Brédas, D. Beljonne, V. Coropceanu, J. Cornil, Chem. Rev. 2004, 104, 4971–5003;
- 5cM. A. Fox, Acc. Chem. Res. 1999, 32, 201–207.
- 6
- 6aH. Imahori, Y. Sakata, Adv. Mater. 1997, 9, 537–546;
- 6bN. Martín, L. Sánchez, B. Illescas, I. Pérez, Chem. Rev. 1998, 98, 2527–2548;
- 6cD. M. Guldi, N. Martín, J. Mater. Chem. 2002, 12, 1978–1992;
- 6dD. M. Guldi, Chem. Soc. Rev. 2002, 31, 22–36;
- 6eJ.-F. Nierengarten, Top. Curr. Chem. 2003, 228, 87–110;
- 6fJ. L. Segura, N. Martín, D. M. Guldi, Chem. Soc. Rev. 2005, 34, 31–47;
- 6gD. M. Guldi, G. M. A. Rahman, C. Ehli, V. Sgobba, Chem. Soc. Rev. 2006, 35, 471–487.
- 7For recent examples, see:
- 7aC. M. Atienza, G. Fernández, L. Sánchez, N. Martín, I. Sá Dantas, M. W. Wienk, R. A. J. Janssen, G. M. A. Rahman, D. M. Guldi, Chem. Commun. 2006, 514–516;
- 7bG. Kodis, Y. Terazono, P. A. Liddell, J. Andréasson, V. Garg, M. Hambourger, T. A. Moore, A. L. Moore, D. Gust, J. Am. Chem. Soc. 2006, 128, 1818–1827;
- 7cL. Sánchez, M. Sierra, N. Martín, D. M. Guldi, M. W. Wienk, R. A. J. Janssen, Org. Lett. 2005, 7, 1691–1694;
- 7dT. Oike, T. Kurata, K. Takimiya, T. Otsubo, Y. Aso, H. Zhang, Y. Araki, O. Ito, J. Am. Chem. Soc. 2005, 127, 15372–15373;
- 7eD. M. Guldi, F. Giacalone, G. de la Torre, J. L. Segura, N. Martín, Chem. Eur. J. 2005, 11, 7199–7210;
- 7fL. Sánchez, M. A. Herranz, N. Martín J. Mater. Chem. 2005, 15, 1409–1421;
- 7gR. S. Iglesias, C. G. Claessens, T. Torres, G. M. A. Rahman, D. M. Guldi, Chem. Commun. 2005, 2113–2115;
- 7hK. Ohkubo, H. Kotani, J. Shao, Z. Ou, K. M. Kadish, G. Li, R. K. Pandey, M. Fujitsuka, O. Ito, H. Imahori, S. Fukuzumi, Angew. Chem. 2004, 116, 871–874;
10.1002/ange.200352870 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 853–856;
- 7iF. Giacalone, J. L. Segura, N. Martín, D. M. Guldi, J. Am. Chem. Soc. 2004, 126, 5340–5341;
- 7jM. Gutierrez-Nava, G. Accorsi, P. Masson, N. Armaroli, J. F. Nierengarten, Chem. Eur. J. 2004, 10, 5076–5086.
- 8
- 8aF. Diederich, M. Gómez-López, Chem. Soc. Rev. 1999, 28, 263–278;
- 8bL. Sánchez, N. Martín, D. M. Guldi, Angew. Chem. 2005, 117, 5508–5516; Angew. Chem. Int. Ed. 2005, 44, 5374–5382;
- 8cSpecial Issue on Supramolecular Chemistry of Fullerenes (Eds.: N. Martín, J. F. Nierengarten), Tetrahedron 2006, 62, 1905–2132;
- 8dU. Hahn, M. Elhabiri, A. Trabolsi, H. Herschbach, E. Leize, A. Van Dorsselaer, A.-M. Albrecht-Gary, J.-F. Nierengarten, Angew. Chem. 2005, 117, 5472–5475; Angew. Chem. Int. Ed. 2005, 44, 5338–5341;
- 8eN. D. McClenaghan, Z. Grote, K. Darriet, M. Zimine, R. M. Williams, L. De Cola, D. M. Bassani, Org. Lett. 2005, 7, 807–810;
- 8fJ. L. Sessler, J. Jayawickramarajah, A. Gouloumis, T. Torres, D. M. Guldi, S. Maldonado, K. J. Stevenson, Chem. Commun. 2005, 1892–1894.
- 9
- 9aJ. P. Kirby, J. A. Roberts, D. G. Nocera, J. Am. Chem. Soc. 1997, 119, 9230–9236;
- 9bN. H. Damrauer, J. M. Hodgkiss, J. Rosenthal, D. G. Nocera, J. Phys. Chem. B 2004, 108, 6315–6321.
- 10
- 10aM. Haj-Zaroubi, N. W. Mitzel, F. P. Schmidtchen, Angew. Chem. 2002, 114, 111–114;
10.1002/1521-3757(20020104)114:1<111::AID-ANGE111>3.0.CO;2-P Google ScholarAngew. Chem. Int. Ed. 2002, 41, 104–107;10.1002/1521-3773(20020104)41:1<104::AID-ANIE104>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 10bV. Král, F. P. Schmidtchen, K. Lang, M. Berger, Org. Lett. 2002, 4, 51–54;
- 10cM. Berger, F. P. Schmidtchen, Angew. Chem. 1998, 110, 2840–2842;
10.1002/(SICI)1521-3757(19981002)110:19<2840::AID-ANGE2840>3.0.CO;2-I Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2694–2694.10.1002/(SICI)1521-3773(19981016)37:19<2694::AID-ANIE2694>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 11J. Otsuki, K. Iwasaki, Y. Nakano, M. Itou, Y. Araki, O. Ito, Chem. Eur. J. 2004, 10, 3461–3466.
- 12M. Segura, L. Sánchez, J. de Mendoza, N. Martín, D. M. Guldi, J. Am. Chem. Soc. 2003, 125, 15093–15100.
- 132D ROESY spectrometry was performed to confirm peak assignments in the 1D 1H NMR spectrum of 1 a.
- 14The high stability of the 1 a⋅2 complex prevents reliable determination of the association constants using NMR techniques; see Ref. [9].
- 15H. Imahori, H. Yamada, D. M. Guldi, Y. Endo, A. Shimomura, S. Kundu, K. Yamada, T. Okada, Y. Sakata, S. Fukuzumi, Angew. Chem. 2002, 114, 2450–2453;
10.1002/1521-3757(20020703)114:13<2450::AID-ANGE2450>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2344–2347.10.1002/1521-3773(20020703)41:13<2344::AID-ANIE2344>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar