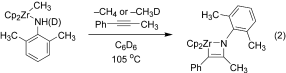Zirconium-Mediated Conversion of Amides to Nitriles: A Surprising Additive Effect†
This work was supported by the National Institutes of Health (GM-25459) and by an NIH post-doctoral fellowship to R.T.R. We thank Dr. Fred Hollander and Dr. Allen Oliver of the UC Berkeley CHEXRAY facility for the X-ray crystal structure determination.
Graphical Abstract
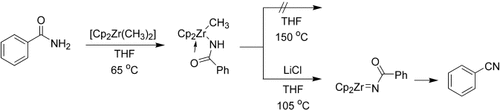
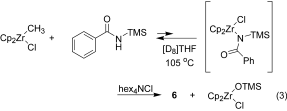
Chloride coordination is the key: Dimethylzirconocene reacts with amides to form methylzirconium amide complexes. On heating, in the presence of a chloride source, these compounds are converted into N-acylimidozirconocene complexes that react intramolecularly to form the corresponding nitrile compounds (see scheme; Cp=C5H5). Mechanistic studies reveal that chloride coordination to zirconium is required for this transformation to occur.