A New Type of Trinuclear Oxoiron(III) Cluster†
Jean-Marc Vincent
L. E. D. S. S., URA 332, Université J. Fourier, BP53X, F-38052 Grenoble (France). Telefax: Int. code + 76 51 43 82
Search for more papers by this authorStéphane Ménage
L. E. D. S. S., URA 332, Université J. Fourier, BP53X, F-38052 Grenoble (France). Telefax: Int. code + 76 51 43 82
Search for more papers by this authorJean-Marc Latour
Département de Recherche Fondamentale sur la Matière Condensée, Laboratoire SESAM/CC (URA 1194), Centre d'Etudes de Grenoble
Search for more papers by this authorAzzedine Bousseksou
Laboratoire de Chimie de Coordination du CNRS, UPR 8241, Toulouse
Search for more papers by this authorJean-Pierre Tuchagues
Laboratoire de Chimie de Coordination du CNRS, UPR 8241, Toulouse
Search for more papers by this authorAndré Decian
Laboratoire de Chimie des Métaux de Transition et Catalyse, URA 424, Université Strasbourg I
Search for more papers by this authorCorresponding Author
Prof. Marc Fontecave
L. E. D. S. S., URA 332, Université J. Fourier, BP53X, F-38052 Grenoble (France). Telefax: Int. code + 76 51 43 82
L. E. D. S. S., URA 332, Université J. Fourier, BP53X, F-38052 Grenoble (France). Telefax: Int. code + 76 51 43 82Search for more papers by this authorJean-Marc Vincent
L. E. D. S. S., URA 332, Université J. Fourier, BP53X, F-38052 Grenoble (France). Telefax: Int. code + 76 51 43 82
Search for more papers by this authorStéphane Ménage
L. E. D. S. S., URA 332, Université J. Fourier, BP53X, F-38052 Grenoble (France). Telefax: Int. code + 76 51 43 82
Search for more papers by this authorJean-Marc Latour
Département de Recherche Fondamentale sur la Matière Condensée, Laboratoire SESAM/CC (URA 1194), Centre d'Etudes de Grenoble
Search for more papers by this authorAzzedine Bousseksou
Laboratoire de Chimie de Coordination du CNRS, UPR 8241, Toulouse
Search for more papers by this authorJean-Pierre Tuchagues
Laboratoire de Chimie de Coordination du CNRS, UPR 8241, Toulouse
Search for more papers by this authorAndré Decian
Laboratoire de Chimie des Métaux de Transition et Catalyse, URA 424, Université Strasbourg I
Search for more papers by this authorCorresponding Author
Prof. Marc Fontecave
L. E. D. S. S., URA 332, Université J. Fourier, BP53X, F-38052 Grenoble (France). Telefax: Int. code + 76 51 43 82
L. E. D. S. S., URA 332, Université J. Fourier, BP53X, F-38052 Grenoble (France). Telefax: Int. code + 76 51 43 82Search for more papers by this authorThis research was supported by the Centre National de la Recherche Scientifique (URA 332 and GDR Métalloprotéines et Analogues de Synthèses). We are grateful to Professor F. Varret for helpful discussions.
Graphical Abstract
References
- 1(a) D. M. Kurtz, Jr., Chem. Rev. 1990, 90, 585. (b) J. S. Lippard, Angew. Chem. 1988, 100, 353. Angew. Chem. Int. Ed. Engl. 1988, 27, 344. (c) K. S. Hagen, Angew. Chem. Int. Ed. Engl. 1992, 104, 1036 and Angew. Chem. Int. Ed. Engl. 1992, 31, 1010, and references therein.
- 2(a) L. Que, Jr., A. E. True, Prog. Inorg. Chem. 1990, 38, 97. (b) J. Sanders-Loehr in Iron Carriers and Iron Proteins (Ed.: T. M. Loehr), VCH, New York, 1989, p. 373.
- 3(a) J. B. Vincent, J. C. Huffman, G. Christou, Q. Li, M. A. Nanny, D. N. Hendrickson, R. H. Fong, R. H. Fish, J. Am. Chem. Soc. 1988, 110, 6898. (b) R. H. Fish, M. S. Konings, K. J. Oberhausen, R. H. Fong, W. M. Yu, G. Christou, J. B. Vincent, D. K. Coggin, R. M. Buchanan, Inorg. Chem. 1991, 30, 3002; and references therein. (c) N. Kitajima, H. Fukui, Y. Moro-oka, J. Chem. Soc. Chem. Commun. 1998, 485. (d) R. A. Leising, J. Kim, M. A. Pérez, L. Que, Jr., J. Am. Chem. Soc. 1993, 115, 9524. (e) S. Ménage, J. M. Vincent, C. Lambeaux, G. Chottard, A. Grand, M. Fontecave, Inorg. Chem. 1993, 32, 4766.
- 4 A. Treffry, J. Hirzmann, S. J. Yewdall, P. M. Harrison, FEBS Lett. 1992, 302, 108.
- 5 Abbreviations used: OAc = acetate, phen = 1,10-phenanthroline, Me2bpy = 4,4′-dimethyl-2,2′-bipyridine, EtOAc = ethyl acetate, T1,4DMIP = tris(1,4-dimethylimidazol-2-yl)phosphane.
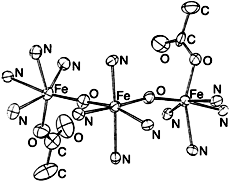
- 6 Diffraction-quality red crystals (0.4 × 0.2 × 0.1 mm) were obtained from ethyl acetate/acetonitrile. The complex 2 · EtOAc crystallizes in the monoclinic system, space group P21/c, with a = 13.336(4), b = 22.804(6), c = 25.181(7) Å, β = 92.41(2), V = 7668.7 Å3, Z = 4. 5299 Unique observed reflections, for which I > 3.0σ(I), were collected at 293K with Mokα radiation (λ = 0.7 1069 Å) to 20max = 26° on an Enraf-Nonius CAD4F X-ray diffractometer. The structure was solved by direct methods and refined to R = 0.074, Rw = 0.099. Further details of the crystal structure investigation may be obtained from the Director of the Cambridge Crystallographic Data Centre, 12 Union Road, GB-Cambridge CB21EZ (UK), on quoting the full journal citation.
- 7 V. Vonkai, G. Newton, D. M. Kurtz, Inorg. Chem. 1992, 31, 34.
- 8 N. Kitajima, H. Amagai, N. Tamura, M. Ito, Y. Moro-oka, K. Heerwergh, A. Pénicaud, R. Mathur, C. A. Reed, P. D. Boyd, Inorg. Chem. 1993, 32, 3583.
- 9 Magnetic susceptibility data were measured on 17.44-mg microcrystalline sample with a Quantum Design MPMS SQUID magnetometer. Data were collected over the temperature range 2–300 K at field strengths of 3,2,1, and 0.5 Tesla.
- 10 S. M. Gorun, S. J. Lippard, Inorg. Chem. 1991, 30, 1625.
- 11 EPR spectra of 2 in the solid state and in acetonitrile were similar, which shows that 2 does not dissociate in solution. In contrast to the 1H NMR spectra of [Fe3(μ-OH)2(μ-OAc)4(T1,4DMIP)2](PF6<)3 and other oxoiron(III) complexes like 1, the spectrum of 2 is very complex with resonances in the range 90 ≥ δ ≥ −60. The signal of the methyl acetato ligand has been assigned by comparison with the trifluoroacetato derivative (δCH3 = 39). Also resonance Raman spectra show a Fe–O vibration from a linear Fe-O-Fe unit.
- 12 J. J. Girerd, G. C. Papaefthymiou, A. D. Watson, E. Gamp, K. S. Hagen, N. Edelstein, R. B. Frankel, R. H. Holm, J. Am. Chem. Soc. 1984, 106, 5941.
- 13(a) W. R. Hagen, Adv. Inorg. Chem. 1992, 38, 165. (b) S. Wickman, J. Chem. Phys. 1965, 42, 2113.
- 14 Mössbauer measurements were made with conventional constant-acceleration spectrometer with a 50-mCi source of 57Co (Rh matrix). The absorber was a microcrystalline powder sample (120 mg) of 2. Variable-temperature spectra were obtained in the 4–300 K range by using a MD 306 Oxford cryostat.
- 15
N. N. Greenwood,
T. C. Gibbs, in
Mössbauer Spectroscopy,
Chapman & Hall, New York,
1971.
10.1007/978-94-009-5697-1 Google Scholar
- 16 To prove these relaxation phenomena it is necessary to reach a static state and observe the internal magnetic field. This can be obtained by applying a weak magnetic field. When an external magnetic field of 2 Tesla was applied at 7 K, the magnetic fluctuations were frozen, and analysis of the resulting Zeeman spectrum allowed us to measure an internal effective magnetic field of roughly 3.9 T.