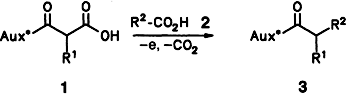Diastereoselective Coupling of Anodically Generated Radicals Bearing Chiral Amide Groups†‡
Dipl.-Chem. Bruno Klotz-Berendes
Organisch-chemisches Institut der Universität, Corrensstrasse 40, D-48149 Münster (Germany). Telefax: Int. code + (251)83-9772
Search for more papers by this authorCorresponding Author
Prof. Dr. Hans J. Schäfer
Organisch-chemisches Institut der Universität, Corrensstrasse 40, D-48149 Münster (Germany). Telefax: Int. code + (251)83-9772
Organisch-chemisches Institut der Universität, Corrensstrasse 40, D-48149 Münster (Germany). Telefax: Int. code + (251)83-9772Search for more papers by this authorDr. Matthias Grehl
Organisch-chemisches Institut der Universität, Corrensstrasse 40, D-48149 Münster (Germany). Telefax: Int. code + (251)83-9772
X-ray Structure analyses
Search for more papers by this authorDr. Roland Fröhlich
Organisch-chemisches Institut der Universität, Corrensstrasse 40, D-48149 Münster (Germany). Telefax: Int. code + (251)83-9772
X-ray Structure analyses
Search for more papers by this authorDipl.-Chem. Bruno Klotz-Berendes
Organisch-chemisches Institut der Universität, Corrensstrasse 40, D-48149 Münster (Germany). Telefax: Int. code + (251)83-9772
Search for more papers by this authorCorresponding Author
Prof. Dr. Hans J. Schäfer
Organisch-chemisches Institut der Universität, Corrensstrasse 40, D-48149 Münster (Germany). Telefax: Int. code + (251)83-9772
Organisch-chemisches Institut der Universität, Corrensstrasse 40, D-48149 Münster (Germany). Telefax: Int. code + (251)83-9772Search for more papers by this authorDr. Matthias Grehl
Organisch-chemisches Institut der Universität, Corrensstrasse 40, D-48149 Münster (Germany). Telefax: Int. code + (251)83-9772
X-ray Structure analyses
Search for more papers by this authorDr. Roland Fröhlich
Organisch-chemisches Institut der Universität, Corrensstrasse 40, D-48149 Münster (Germany). Telefax: Int. code + (251)83-9772
X-ray Structure analyses
Search for more papers by this authorElectroorganic Syntheses, Part 60. This work was supported by the Fonds der Chemischen Industrie and the EC Human Capital and Mobility Programme (no. ERBCHRXCT 920073). Part 59: C. Zielinski, H. J. Schäfer, Tetrahedron Lett. 1994, 35, 5621–5624.
Dedicated to Professor Wolfgang Lüttke on the occasion of his 75th birthday
Graphical Abstract
References
- 1
H. J. Schäfer,
Top. Curr. Chem.
1990,
152, 91–151.
10.1007/BFb0034365 Google Scholar
- 2 L. Eberson, G. Ryde-Petterson, Acta Chem. Scand. 1973, 27, 1159–1161. L. Eberson, K. Nyberg, R. Servin, Acta Chem. Scand. Ser. B 1976 30, 906–907. G. E. Hawkes, J. H. P. Utley, G. B. Yates, J. Chem. Soc. Perkin Trans. 2 1976, 1709–1716.
- 3 D. Seebach, P. Renaud, Angew. Chem. 1986, 98, 836–838. Angew. Chem. Int. Ed. Engl. 1986, 25, 843–845.
- 4 P. Wessig, P. Wettstein, B. Giese, M. Neuburger, M. Zehnder, Helv. Chem. Acta 1994, 77, 829–837.
- 5 S. Masamune, R. P. Short, R. M. Kennedy, J. Org. Chem. 1989, 54, 1755–1756.
- 6(a) E. L. Eliel, R. O. Hutchins, Sr. M. Knoeber, Org. Synth. 1970, 50, 38–42. (b) F. W. Nader, A. Brecht, S. Kreisz, Chem. Ber. 1986, 119, 1196–1207.
- 7 D. Evans, J. R. Gage, Org. Synth. 1990, 68, 77–82.
- 8 In alkaline media only the oxazolidone residue is hydrolyzed; with pig liver esterase and in acid media no conversion occurs.
- 9 M. S. Newman, A. Kunter, J. Am. Chem. Soc. 1951, 73, 4199–4204.
- 10 G. Helmchen, A. Selim, D. Dorsch, I. Taufer, Tetrahedron Lett. 1983, 24, 3213–3216.
- 11 Available from Merck.
- 12 Synthesized as a new auxiliary in analogy to the method of N. A. Porter, I. J. Rosenstein, R. A. Breyer, J. D. Brunke, W. Wu. A. T. McPhail, J. Am. Chem. Soc. 1992, 114, 7664–7676.
- 13 Obtained as a new auxiliary by a modification of a method for preparing oxazolidones by P. Michon, A. Rassat. Bull. Soc. Chim. Fr. 1971, 3561–3567.
- 14 For the independent synthesis of 5a the lithium salt of (S)-3-(1-oxohexyl)-4-(phenylmethyl)-2-oxazolidinone was alkylated with benzyl bromide with 98% de according to the method of D. A. Evans, M. D. Ennis, D. J. Mathre, J. Am. Chem. Soc. 1982, 104, 1737–1739. On the grounds of the mechanism of the reaction, the newly formed steregenic center has (R) configuration. Removal of the auxiliary without racemization was carried out according to the method of D. A. Evans, J. Gage, Org. Synth. 1990, 68, 83–91. Auxiliary A was subsequently coupled without racemization to the acid chloride. The product is identical with 5a according to gas chromatography and 1H NMR spectroscopy.
- 15 Crystal structure data for 6k: C21H37NO3S, M = 383.58, crystal dimensions 0.3 × 0.25 × 0.2mm, a = 8.718(1), b = 11.861(3), c = 11.137(2) Å, β = 108.02(1)°, V = 1095.1(4) Å3, ρcalcd = 1.163 gcm−3, μ = 14.6 cm−1, Z = 2, monoclinic, space group P21 (no. 4). Enraf-Nonius CAD4 diffractometer, λ = 1.54178 Å, T = 223 K, ω–2θ scan, 2455 measured reflections (± h, – k, + l), 2337 independent and 2151 observed [I ≥ 2σ( I)] reflections, 244 refined parameters, R = 0.068, w R2 = 0.172, Flack parameter 0.00(3), max. residual electron density 0.85(–0.61)e Å3, solution by direct methods, hydrogen atoms in calculated positions. Crystal structure data for 6n (reaction of 1e with 2c): C21H31NO3, M = 345.47, crystal dimenstions 0.4 × 0.4 × 0.4mm, a = 9.501(2), b = 11.489(2), c = 18.756(4)Å, V = 2047.3(7)Å3, ρcalcd = 1.121 gcm−1, μ = 5.9 cm−1, Z = 4, orthorhombic, space group P212121 (no. 19), Enraf-Nonius CAD4 diffractometer, λ = 1.54178 Å, T = 293 K, ω–2θ scan, 4802 measured reflections (– h, + k, ± l), 4210 independent and 4028 observed [ l ≥ 2θ(1)] reflections, 233 refined parameters, R = 0.042, wR2 = 0.116, Flack parameter −0.2(2), max, residual electron density 0.21(–0.14) e Å3, solution by direct methods, hydrogen atoms in calculated positions. Crystal data for 61: C23H37NO2, M = 359.54, crystal dimensions 0.25 × 0.2 × 0.4 mm, a = 9.182(4), b = 13.564(4), c = 17.965(2)Å, V = 2237.4(12) Å3, ρcalcd = 1.067 gcm−3, μ = 5.1 cm−1, Z = 4, orthorhombic, space group P212121 (no. 4), Enraf-Nonius CAD4 diffractometer, λ = 1.54178 Å, T = 223 K, ω–2θ scan, 4213 measured reflections (+ h, − k, ± l), 3793 independent and 2187 observed [I > 2θ( I)] reflections, 244 refined parameters R = 0.061, wR2 = 0.144, Flack parameter −2.0(6), max, residual electron density 0.28(–0.16) e Å3, solution by direct methods, hydrogen atoms in calculated positions. Programs used: SHELX-86, SHELX-93, SCHAKAL-92. Further details of the crystal structure investigation may be obtained from the Fachinformationszentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen (Germany) on quoting the depository numbers CSD-401246 for 6k, CSD-401247 for 6n, and CSD-401248 for 61.
- 16(a) W. Strub, E. Roduner, H. Fischer, J. Phys. Chem. 1987, 91, 4379–4383. (b) N. A. Porter, I. J. Rosenstein, R. A. Breyer, J. D. Bruhnke, W.-X. Wu, A. T. McPhail, J. Am. Chem. Soc. 1992, 114, 7664–7676.
- 17For applications of auxiliary A in diastereoselective radical additions see: N. A. Porter, D. M. Scott, I. J. Rosenstein, B. Giese, H. G. Zeitz, A. Veit, J. Am. Chem. Soc. 1991, 113, 1791–1799. N. A. Porter, D. M. Scott, A. T. McPhail, Tetrahedron Lett. 1990, 31, 1679–1682.
- 18For applications of auxiliary E in diasteroselective radical additions see: D. Curran, W. Shen, W. Zhang, T. A. Heffner, J. Am. Chem. Soc. 1990, 112, 6738–6740.
- 19 For applications of oxazolidines as chiral auxiliaries in diasteroselective radical additions see: N. A. Porter, I. J. Rosenstein, R. A. Breyer, J. D. Bruhnke, W. Wu, J. Am. Chem. Soc. 1991, 113, 7788.
- 20(a) N. A. Porter, B. Giese, D. P. Curran, Acc. Chem. Res. 1991, 24, 296–204. (b) W. Smadja, Synlett 1994, 1–26.
- 21 K. K. Ingold in Free Radicals, Vol. 1 (Ed.: J. K. Kochi), Wiley, New York, 1973, p. 37.