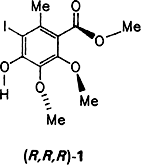
Methyl 4-Hydroxy-5-iodo-2,3-dimethoxy-6-methylbenzoate: The Aromatic Fragment of Calichemicin γ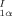 . Synthesis, X-Ray Crystallographic Analysis, and Properties†
. Synthesis, X-Ray Crystallographic Analysis, and Properties†
This work was supported by Lederle Laboratories, Pearl River, NY, and by the University of Pennsylvania. Stimulating and helpful discussions with Dr. May Lee and Dr. Ving Lee (Lederle Laboratories), Dr. David Eaton (Du Pont de Nemours, Experimental Station, Wilmington, DE), Dr. R. Huffman (Indiana University), Prof. Jay Siegel (University of California, San Diego), and Prof. Kurt Mislow (Princeton University) are acknowledged. We also thank Dr. George Furst and John Dykins of our Department for their excellent NMR and mass spectroscopic assistance and useful comments.
Graphical Abstract
The biologically highly active calichemicin γ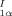 contains the benzoate 1 — glycosylated on the OH group and linked through the ester group (S instead of O), via sugar moieties, to other unusual organic fragments. Compound 1 has now been synthesized in five steps from 3,4,5-trimethoxytoluene and has proven to be very interesting. It undergoes spontaneous enantiomer resolution upon crystallization and exhibits second harmonic generation activity.
contains the benzoate 1 — glycosylated on the OH group and linked through the ester group (S instead of O), via sugar moieties, to other unusual organic fragments. Compound 1 has now been synthesized in five steps from 3,4,5-trimethoxytoluene and has proven to be very interesting. It undergoes spontaneous enantiomer resolution upon crystallization and exhibits second harmonic generation activity.