Azirineimine as a Structural Unit in the Side Chain of a Hexofuranose†
We thank Dr. I. Lauterwein, Organisch-chemisches Institut der Universität Münster (FRG), for invaluable assistance in interpreting the nuclear resonance spectra; we thank the NMR-department for carrying out the measurements.
Graphical Abstract
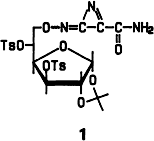
Azirineimine, formally an HCN dimer, was obtained for the first time as derivative 1. Compound 1 is formed upon reaction of 1,2-O-isopropylidene-3,5,6-tri-O-p-toluenesulfonyl-α-D-glucose with KCN in nitromethane/water under phase-transfer conditions. Theoretical studies have shown that azirineimine may have played a role in the prebiotic formation of organic compounds.