Advances in the Design of Photoactivatable Metallodrugs: Excited State Metallomics
Abstract
Photoactivatable metal complexes offer the prospect of novel drugs with low side effects and new mechanisms of action to combat resistance to current therapy. We highlight recent progress in the design of platinum, ruthenium, iridium, gold and other transition metal complexes, especially for applications as anticancer and anti-infective agents. In particular, understanding excited state chemistry related to identification of the bioactive species (excited state metallomics/pharmacophores) is important. Photoactivatable metallodrugs are classified here as photocatalysts, photorelease agents and ligand-activated agents. Their activation wavelengths, cellular mechanisms of action, experimental and theoretical metallomics of excited states and photoproducts are discussed to explore new strategies for the design and investigation of photoactivatable metallodrugs. These photoactivatable metallodrugs have potential in clinical applications of Photodynamic Therapy (PDT), Photoactivated Chemotherapy (PACT) and Photothermal Therapy (PTT).
1 Introduction
Metals play a vital role in many biological processes and have long been widely explored in medicines.1 The development of modern metallodrugs was stimulated by U.S. Food and Drug Administration (FDA) approval of cisplatin for the treatment of testicular cancer in 1978, which is still a major drug in cancer chemotherapy.1d Although organic drugs dominate the pharmaceutical market, metallodrugs, not only based on Pt(II), are emerging due to their rich biological and chemical diversities.1d, 2 Metallodrugs are often prodrugs, activated by ligand exchange or redox reactions before reaching their cellular targets.1e Both the metal and its ligands can play important roles in the biological activity. The activation triggers for metallodrugs can be endogenous or exogenous stimuli. Exogenous stimuli that can be applied externally to patients provide accurate remote control over prodrug activation spatially and temporally to reduce side effects and systemic toxicity.1e Light, a safe and non-invasive exogenous stimulus, has been applied in the treatment of various diseases.3
The beginning of modern phototherapy was the treatment of lupus vulgaris using concentrated light irradiation by Finsen.4 The first PDT agent, Photofrin®, a mixture of oligomeric HpD (hematoporphyrin) derivatives, was approved in 1993 to treat bladder cancer in Canada and is still being investigated in clinic trials.5 Metal-based photosensitizers Pd(II) complex Tookad® soluble approved by European Medicines Agency (EMA), Al(III) complex Photosens® approved in Russia, Lu(III) complex Antrin®, Sn(IV) complex Purlytin® and Zn(II) complex CGP55847 have all entered clinical trials, and are based on a tetrapyrrole structure(Figure 1).3a, 5c They can be activated using red or NIR light. The polypyridyl Ru(II) complex TLD1433 is now in phase II clinical trials for non-muscle invasive bladder cancer (NMIBC) and is activatable by green light.6 Despite the clinical success of PDT agents, their applications are usually limited due to their oxygen-dependent mechanism of action and the hypoxic nature of tumor microenvironments (TME). Strategies to improve PDT efficacy under hypoxia have been investigated, including increasing the oxygen concentration in tumors or decreasing oxygen consumption during PDT.7 In contrast, PACT is not dependent on oxygen for photocytotoxicity and thus promising for the treatment of hypoxic tumors, but is still at an early stage of development.
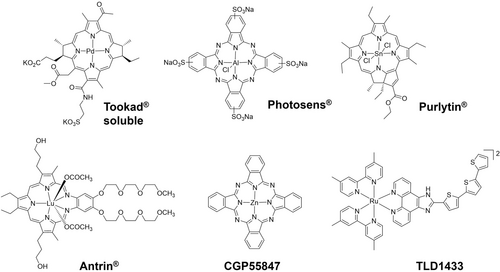
Photoactivatable metallodrugs that have entered clinical trials.
The absorption of light by metal complexes leads to the population of excited states with higher energy levels. These excited state molecules show dramatic differences in electronic distributions and reactivities compared to their ground states. Typical electronic transitions of metal complexes include metal-centered (MC, also referred as ligand field (LF)), ligand-centered (LC, also called intraligand (IL)), or involve both the metal and the ligands: metal-to-ligand charge transfer (MLCT) and ligand-to-metal charge transfer (LMCT), Figure 2a.3, 6 In addition, charge transfer can involve two different ligands as ligand-to-ligand charge transfer (LLCT), or two different metals in multimetallic complexes as metal-to-metal charge transfer (MMCT). Upon light irradiation, no ligand dissociation is usually observed for PDT agents, while for PACT a change in the coordination sphere is expected. Hence, the population of excited metal-containing states plays a crucial role in the reactivity of these two therapeutic processes.
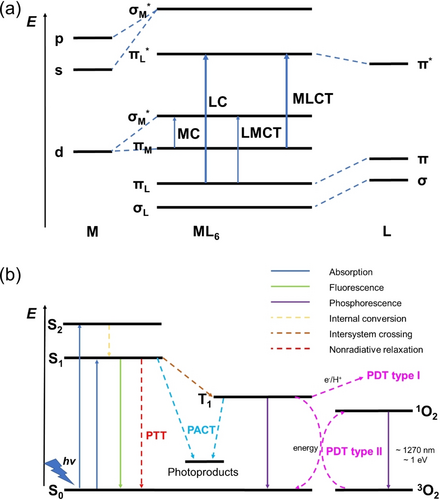
(a) Molecular orbital diagram representing various types of electronic transitions in octahedral complexes; (b) Reactive pathways for photoactivatable metallodrugs (Jabłonski diagram). Part (b) is reproduced from ref. 3c with permission.
Metal complexes in the excited state undergo not only radiative processes, e.g. fluorescence and phosphorescence, but also nonradiative processes, e.g. intersystem crossing (ISC), internal conversion (IC), intramolecular vibrational redistribution and solvation dynamics (reorganization of solvent shells), ultimately leading back to the ground-states (Figure 2b).3c Thus, investigations of excited state metallomics play an important role in understanding mechanisms of action and optimization of the design of future photoactivatable metallodrugs.
Here we summarize recent advances in the design of photoactivable metallodrugs, both for PDT and PACT, mainly in anticancer therapy. Photoactivable metallodrugs possess a wide range of structures with diverse oxidation states, coordination numbers, and geometries to provide unique photophysical and photochemical properties and novel mechanisms of action.3 Metals allow quantification and localization of the agents using a range of methods, such as inductively-coupled-plasma (ICP) optical emission spectroscopy (OES) and mass spectrometry, X-ray fluorescence (XRF), X-ray absorption near edge structure (XANES), secondary ion mass spectrometry (SIMS) and positron emission tomography (PET).1d The following aspects will be discussed for photoactivable metallodrugs: photoactivation mechanisms, activation wavelengths, cellular mechanisms of action, and metallomics. In particular, the exploration of excited state metallomics in the development of photoactivable metallodrugs, and opportunities and challenges for the clinical translation of photoactivable metallodrugs will be explored.
2 Photoactivation Mechanisms
According to the active factors, phototherapy is typically classified as PDT, PACT and PTT, with photoactivation mechanisms including photocatalysis, photorelease, photoactivation of ligands, and a combination of two or more mechanisms (Figure 3). Generally, PDT and PTT are based on photocatalysis, while PACT involves photorelease and photoactivation of ligands.
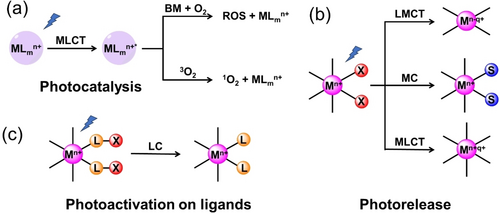
Photoactivation mechanisms for metallodrugs (M: metal; L: ligand; BM: biomolecules; S: solvent; X: photoactive moiety).
2.1 Photocatalysis
PDT has been increasingly applied for treating a wide variety of cancers such as lung,8 skin melanoma and non-melanoma,9 and prostate,10 as an auxiliary method to improve life quality of patients, and often used in combination with other therapies, e.g. surgery, chemotherapy, radiotherapy, and immunotherapy.11 Photocatalysts in PDT can generate cytotoxic reactive oxygen species (ROS) from ambient oxygen under optical excitation, but are not toxic in the dark, thus providing spatial, temporal and light-dose control of the treatment to reduce damage to the neighboring tissue.3 Notably, due to their catalytic nature, only small doses of photocatalysts are needed.
Upon irradiation, the photocatalyst in its singlet ground state (S0) forms a singlet excited state (S1), followed by an ISC process to reach a triplet excited state (T1), which can generate cytotoxic radicals through electron transfer by interaction with biomolecules or by forming superoxide radicals (Type I pathway), or convert ground-state oxygen (3O2) directly into highly reactive cytotoxic singlet oxygen (1O2) through an energy transfer process (Type II pathway, Figure 2b). Singlet oxygen formation (Type II) is generally considered to be the principal mechanism for PDT, with its high energy of 94 kJ mol−1 (equals to 22 kcal mol−1) for the 1Δg state above the triplet 3Σg ground state.3c, 12 However, due to its dependence on oxygen, its efficacy is limited in hypoxic tumors. Metal-based photocatalysts provide the possibility of generating oxygen-independent ROS, a possible strategy to overcome hypoxia resistance in PDT.13
Apart from the photoactivatable complexes shown in Figure 1, which have entered clinical trials, other Ru(II),14 Ir(III),3b, 14a, 15 Os(II)16 and other metal complexes17 have been developed as photoactivatable photosensitizers for PDT. Their high quantum yields in the formation of triplet excited states and long lifetimes for triplet decay are favorable for interaction with ground state 3O2, resulting in the formation of excited state 1O2.
Ru(II), Ir(III) and Os(II) complexes in particular can act as photocatalysts, since they can adopt various geometries with different steric and electronic properties, have high dark stability and ability to be metabolized, tunable MLCT transition bands for red-shifted absorption wavelengths, suitable redox potentials, solubility, excited state properties with emission at > 600 nm, and a long lifetime of decay that allows differentiation from bioluminescence.18
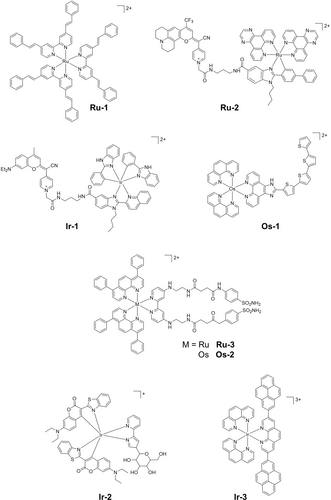
However, the MLCT bands that are characterized by a transition from the HOMO (constituted predominantly by the metal) to the LUMO (located on the ligands), are usually at wavelengths<600 nm, outside the therapeutic window (600–900 nm). Red-shifting such MLCT of metal-based photocatalysts has been explored extensively. Extension of π-conjugated systems is a common strategy to allow activation at longer wavelengths. The chelation of highly conjugated (E,E′)-4,4′-bis-styryl-2,2′-bipyridine ligands with electron-donating substituents to Ru(II), red-shifts the MLCT band by > 50 nm compared to [Ru(bpy)3]2+ to the region 450 nm to 650 nm.19 Ru-1 (Figure 4) and analogues exhibit phototoxicity towards cancer cells under blue (480 nm) and green (540 nm) light due to the formation of singlet oxygen.
Some photocatalytic metal complexes.
Another red-shift strategy is the introduction of chromophores, e.g. small organic chromophores, directly or indirectly coordinated to the metal, which can harvest light to promote formation of the excited state.20 By conjugating a fluorophore to the metal center, the excited state can be achieved by activating MLCT or fluorophore transitions and introduce chromatic screening, e.g. the same molecule can be activated by different wavelengths of light.21 Curcumins,22 coumarins,21 naphthoquinones,23 and hydroxyquinolines24 are commonly used natural organic chromophores. The Ru(II) photocatalyst Ru-2 (Figure 4), which bears a coumarin-containing ligand, shows an absorption band between 400 nm and 700 nm that combines the MLCT and the coumarin absorptions, inducing good photocytotoxicity with 620 nm to 770 nm light.21 Selective energy transfer from 3MLCT* to the coumarin fragment is also observed in the excited state of Ir-1 (Figure 4), a cyclometalated Ir(III) complex with absorption between 500 and 600 nm.25 Notably, Ir-1 only operates under a Type I mechanism with similar IC50 values both in normoxia and hypoxia with blue or green light, indicative of its oxygen-independent photocytotoxicity. Despite the ability of conjugated chromophores to red-shift activation wavelengths by decreasing the LUMO energy, they can also contribute to the HOMO energy. As consequence, the complexes can be more readily oxidized both in the ground and excited states and undergo ligand release.26 In this case, these complexes can undergo photorelease instead of photocatalysis, and thus are discussed in the section 2.2.
Besides red-shifting the absorption band, another strategy to increase the effectiveness of metal-based photocatalysts is the formation of low-lying 3IL* and/or 3ILCT* states. Generally, low-lying excited states are favorable due to spin-orbit coupling between a ligand and a heavy atom, and their long lifetimes lengthen the time of interaction with substrates to form radicals.27 The remarkable photocytotoxicity of TLD1433 is based on this strategy, which facilitates the formation of ROS via Type I and II mechanisms.6 The Os(II) complex bearing four thiophenes rings derived from TLD1433, Os-1 (Figure 4), exhibits a long-lived, low-lying 3ILCT state (τo=3–4 μs) for the photoinduced production of O2⋅− and 1O2. Its phototoxicity indices (PI) are > 106 and > 70 under normoxia and hypoxia, respectively, despite the activation wavelengths (white visible light, 523 nm and 633 nm).28 The elevated phototoxicity under hypoxia can be attributed to a change in the character of the low-lying triplet excited state from 3MLCT* to 3ILCT* with increase in the number of thiophene rings.
Compared to their Ru(II) counterparts, polypyridyl Os(II) complexes absorb longer wavelength light due to the more pronounced heavy atom effect from Os. This leads to a larger spin–orbit coupling, promoting faster intersystem crossing which produces triplet states in high yield.29 When Os is used in the photocatalyst Os-2 with a π-extended ligand (Figure 4), a ca. 30 nm red-shift of the absorbance is observed compared with the Ru(II) analogue, the 1O2 quantum yield decreases from 77 % (Ru-3) to 10 % (Os-2).30 The photocytotoxicity towards MDA-MB-231 cells in normoxia with 540 nm irradiation for Ru-3 was four-times higher than Os-2, directly related to singlet oxygen formation. While, photocytotoxicity with 740 nm irradiation was observable only for Os(II) photocatalysts, rather than their Ru(II) analogue Ru-3 which is related to the longer absorption for Os-2 (Figure 4).
Other than their excellent photostability and high photocytotoxicity, cyclometalated Ir(III) photocatalysts exhibit higher cell membrane permeability due to the presence of phenyl-pyridine ligands with higher lipophilicity than their polypyridyl analogues.15a, 31 The highly aqueous-soluble glycosylated biocompatible cyclometalated Ir(III) complex Ir-2 (Figure 4) shows good photocytotoxicity towards HeLa cells (IC50=0.08 μM) when irradiated with 465 nm light, inducing intracellular ROS production via Type I and Type II mechanisms.32 The strategy of using highly conjugated ligands to form long-lived 3IL/3ILCT excited states can also be employed for Ir(III) complexes. Tris-1,10-phenanthroline Ir(III) complex Ir-3 (Figure 4) has a high photostability and long-lived 3IL/3ILCT excited state (ca. 30 μs) with a high 1O2 quantum yield of 81 %.33 Submicromolar photocytotoxicity (IC50 < 0.70 μM and PI > 250) toward SK-MEL-28 cancer cells under visible light is observed for Ir-3, attributable to the high 1O2 formation through interaction between the (3IL/3ILCT)* state and oxygen.
2.2 Photorelease
In contrast to photostable agents for photocatalysis, metal complexes can release ligands upon irradiation to generate cytotoxic species, an O2-independent mechanism.3, 34 Hence, such complexes hold potential for generating photocytotoxicity under hypoxia.3, 35 Photorelease mechanisms can be classified into two categories: photoinduced charge transfer (photoreduction and photooxidation) and photosubstitution.3 Generally, photoreductive metallodrugs possess dissociative excited states with LMCT nature. Thus, dissociation of ligands by electron transfer to the metal can be induced by irradiation. Pt(IV)35, 36 and Co(III)37 complexes tend to undergo photoreduction to release more labile cytotoxic Pt(II) and Co(II) species. In contrast, Ru(II),38 Rh(III),39 and Re(I)40 complexes undergo photosubstitution by solvent. Photosubstitution for polypyridyl Ru(II) and tricarbonyl Re(I) complexes with sterically bulky ligands typically begins with formation of 1MLCT excited states upon irradiation, and leads to the population of 3MLCT excited states, followed by dissociative triplet metal-centered (3MC) states.3c, 38c 3MC states are favorable for ligand dissociation with substitution by solvent molecules.38c
2.2.1 Photoreduction
Octahedral Pt(IV) complexes have been widely studied as photoreductive agents.35, 36, 41 Early work showed that diiodido-Pt(IV)-ethylenediamines, e.g. Pt-1 (Figure 5), with their strong broad LMCT bands centered at ca. 400 nm with a tail extending out into the visible range, can form bis-guanine Pt(II) adducts upon irradiation with blue light.42 However, they are readily reduced by glutathione in the dark by a mechanism involving attack of the thiolate S on a coordinated iodide, and exhibit low photoselectivity.43
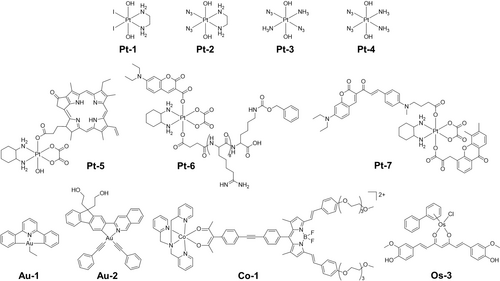
Pt(IV), Au(III) and Co(III) complexes which undergo photoreduction, and a Os(II) complex which undergoes photooxidation.
Second-generation photoactive Pt(IV) complexes [Pt(N3)2(OR1)(OR2)(L1)(L2)] with improved dark stability have been developed by replacing iodido ligands with azides, e.g. Pt-2 (Figure 5).35, 36 Transition metal azido complexes are often light-sensitive.44 Diazido Pt(IV) complexes exhibit low dark cytotoxicity and visible light photocytotoxicity towards various cancer cell lines. In contrast to the preferred cis diam(m)ine geometry for conventional anticancer platinum complexes as indicated by the early structure–activity relationships, diazido Pt(IV) complexes with all trans configuration Pt-3 (Figure 5) exhibit more intense and red-shifted (ca. 30 nm) LMCT bands, increased aqueous solubility and enhanced photocytotoxicity compared to their cis isomers Pt-4 (Figure 5).45 Modifications on equatorial ligands can red-shift the LMCT bands, alter the dark stability, and photocytotoxicity.46 Axial functionalization is a common strategy to modify the photochemistry and photobiology properties of diazido Pt(IV) complexes.35 Photoreleasable axial ligands can significantly alter their activation wavelength, reduction potential, cancer targeting ability, and photocytotoxicity.36 However, most reported diazido Pt(IV) complexes are photoactivatable with blue and green light. For deeper tissue penetration, longer activation wavelengths are required.
Pt(IV) oxaliplatin derivatives with photoabsorbing molecules as axial ligands constitute another class of photoactive Pt(IV) complexes that can undergo photoreduction in the presence of bioreductants to release oxaliplatin and axial ligands.47 By careful selection of photoabsorbing molecules, the activation wavelength can vary from red light to NIR (Pt-5−Pt-7, Figure 5).48 Despite the necessity for bioreductants for the photoactivation of these oxaliplatin derivatives, a more reductive environment in cancer cells than normal cells is favorable for their cancer selectivity.
Cyclometalated Au(III) Complexes are emerging photoreductive metallodrugs, showing photocontrollable thiol-reactivity.49 Au(III)-alkyl complex Au-1 (Figure 5) can undergo photo-induced β-hydride elimination to release the alkyl ligand and form an Au(III)-hydride intermediate that can be quickly converted into a bioactive [Au(III)−S] adduct [Au(C N C)(NAC)] (HC N CH=2,6-diphenylpyridine) in the presence of N-acetylcysteine (NAC) and UVA light.49b The remaining Au-1 can photo-catalytically reduce [Au(C N C)(NAC)] to highly bioactive Au(I) species with excess NAC. Strong inhibition of the selenoenzyme thioredoxin reductase (TrxR), potent cytotoxicity, and strong antiangiogenesis and antitumor activities in vivo were observed for this Au(III)-alkyl complex with one- or two-photon activation. Other than Au(III)-alkyl complexes, cyclometalated Au(III)-alkyne complex Au-2 (Figure 5) is also photoactive and displays oxygen dependent mechanisms of photoactivation.49c Under normoxia, Au-2 photocatalyzes the generation of singlet oxygen and remains photostable upon irradiation. While when oxygen is depleted, the excited states of Au-2 are quenched by physiological thiols to generate Au(I) species as photoreduction products with high quantum yields (φ = 0.63). Intriguingly, the photoreduction of Au-2 can be triggered by external photosensitizers to allow deeper tissue penetration by red light irradiation.
Kinetically-inert low-spin 3d6 Co(III) complexes with low metal-related cytotoxicity are highly susceptible to ligand release on reduction to labile Co(II) complexes, and thus often used as drug chaperons, especially under hypoxia.37c Curcumin and BODIPY dyes can be delivered by photoactive Co(III) chaperons, e.g. Co-1 (Figure 5).37a-37c These Co(III) chaperons can undergo photoreduction to release photosensitizers that can be further activated by light to generate ROS, and result in photocytotoxicity. The activation wavelength of these chaperons is dependent on the delivered photosensitizers.
2.2.2 Photooxidation
Compared with photoreduction, photooxidation of metal centers is less reported. Half-sandwich Os(II)-arene complexes can act as catalytic anticancer drugs, but their photocytotoxicity is little explored. Selective photodissociation of the arene ligand from Os-3 (Figure 5) and oxidation of Os(II) to Os(III) occurs upon blue light or UVA irradiation.50 Os-3 displays remarkable photocytotoxicity towards a range of cancer cell lines, especially cisplatin-resistant cancer cells. It localizes mainly in mitochondria in the dark, but trans-localizes to the nuclei after irradiation, generating DNA and mitochondrial damage.
2.2.3 Photosubstitution
Photosubstitution, photo-induced ligand dissociation with substitution by solvent molecules but no electron transfer, is another important type of photorelease often observed in complexes with sterically bulky ligands.3c, 38c, 51 The binding between metal and solvent in solvated metal species formed in photosubstitution is often relatively weak, and thus such photoproducts can readily attack biomolecules, e.g. DNA bases, leading to cell death. Bioactive small molecules, such as NO and CO, can also be delivered to target sites by these metallodrugs.52 Although photoreduction is regarded as the main photoactivation mechanism for diazido Pt(IV) complexes, non-redox photosubstitution of a photolabile azido ligand by water can form an initial photoactivation step.53 Photosubstitution of chlorido ligands by water in [Rh(III)(phen)2Cl2]Cl, “photo cisplatin”, upon UV irradiation leading to formation of DNA adducts was reported in the 1990s.54
Besides the PDT properties of Ru(II) polypyridyl complexes, bulky ligands can promote photosubstituition in this systems.6, 55 Photosubstitutable polypyridyl Ru(II) complexes were reported in the early 2000s.56 Ru-4 (Figure 6) is an example of the release of a neurocompound, 4-aminopyridine, using visible light which promotes the activation of a leech neuron by blocking its K+ channels.56a In contrast, for Ru-5 the photoproduct, aquated Ru(II) species, is the cytotoxic species (Figure 6), which can bind to ss-DNA and ds-DNA strongly upon irradiation.56b
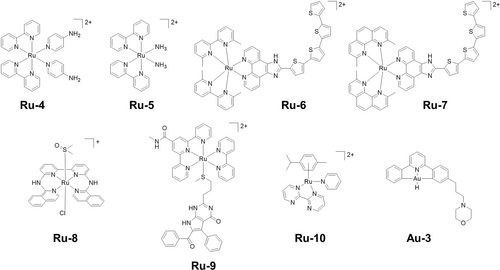
Photosubstitutable Ru(II) and Au(III) complexes.
The introduction of sterically-demanding ligands is a common strategy to increase photo-instability of polypyridyl Ru(II) complexes and promote photosubstitution.38b Bulky ligands increase the distortion of the coordination octahedron and lower the energy of dissociative 3MC excited states, making them accessible from 3MLCT and leading to photosubstitution by solvent molecules.6, 38b Typical bulky ligands used in polypyridyl Ru(II) complexes are ortho-methyl-substituted polypyridines. The degree of rigidity of the bulky ligands is inversely related to the photosubstitution quantum yields of the complexes.57 Upon irradiation, the singlet excited states of methylated derivatives of TLD1433, Ru-6 and Ru-7 (Figure 6), can undergo rapid intersystem crossing to form corresponding triplet excited states.58 Some of these triplet states dissipate their excess energy through photosubstitution or nonradiative decay involving the 3MC states, while others catalyze the generation of 1O2 from the lowest-lying 3IL/3ILCT states. As a result, these derivatives display photocytotoxicity under both normoxia and hypoxia, with PIs as large as > 500,000 and > 5,800, respectively, using broadband visible and monochromatic blue light. The choice of auxiliary ligands also contributes to the photosubstitution of polypyridyl Ru(II) complexes.59
Another class of photosubstitutable polypyridyl Ru(II) complexes contains two monodentate ligands, which can be cis or trans.60 Ru(II) complexes with trans monodentate ligands often contain a quaterpyridine ligand, e.g. Ru-8 (Figure 6).60d Relatively labile monodentate ligands, such as acetonitrile or DMSO, can undergo photosubstitution by water to generate apoptotic photocytotoxicity. Terpyridine (tpy) ligands are also employed in photosubstitutable polypyridyl Ru(II) complexes (Figure 6).61 The restriction of tpy induces a significant distortion of the first coordination sphere of the metal, which significantly lowers the 3MC energy, thus prompting photosubstitution.61, 62 The photoreleased monodentate ligands such as anticancer agents or drug delivery polymers can improve the anticancer efficacy of these Ru(II) complexes, e.g. Ru-9.62
Compared with polypyridyl Ru(II) complexes, the photoactivity of half-sandwich Ru(II) arene complexes has been less studied due to their inherent dark cytotoxicity. However, Ru-10 (Figure 6) with an absorption tail in the 400 nm region based on mixed partially dissociative 1MC−1MLCT transitions, undergoes pyridine photosubstitution by 9-ethylguanine (9-EtG) N7 on visible light irradiation (400–600 nm).63
The hydride ligand in cyclometalated Au(III)-hydride complexes [AuIII(C^N^C)H] can be photosubstituted by NAC, resulting in the formation of Au(III)−S adducts and release of hydrogen upon irradiation.64 The morpholine moiety in Au-3 (Figure 6) allows formation of a cationic species in acidic tumor environments, which can interact with anionic photosensitizers, e.g. Eosin Y and Rose Bengal, tightly, leading to a red-shifted absorption tail in the far-red region and thus red light photocytotoxicity.64b
Examples of Mn(I)-65 and Re(I)-tricarbonyl,60c osmium-peroxo,66 cyclometalated Ir(III)67 and Pt(II)-O,O-bidentate complexes68 have been reported to possess photosubstitution ability. Small gas molecules, e.g. CO or H2, or anticancer-active species, e.g. O2⋅− or curcumin, can be released upon irradiation, accompanied by uncaged metal species for synergistic therapy.
2.3 Photoactivation on ligands
Ligands can not only alter the photoactivation of metal complexes by modulating their absorption and emission, and imposing steric strain, but also generate cytotoxic species by their own photocleavage or photoswitching.3c
Conjugation of a nuclear localization sequence (NLS) peptide to a tricarbonyl Re(I) complex Re-1 (Figure 7) through a photolabile protecting group, can promote delivery selectively to cancer cells.40b Twenty-five percent of Re from Re-1 accumulates in the nuclei and induces severe cell stress in the dark and upon irradiation.
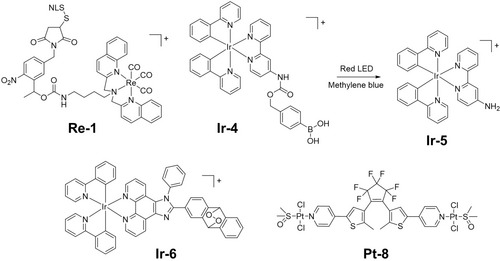
Metal complexes which can undergo photocleavage or act as photoswitches.
Weakly toxic boronic acid-caged Ir(III) complex Ir-4 (Figure 7) can be converted into bioactive Ir-5 (Figure 7) in the presence of red light and methylene blue (MB). The phenyl boronic acid fragment can be photo-oxidized to generate a phenyl radical that can rapidly capture oxygen under hypoxia to remove the boron moiety and release cytotoxic species.69 The concept was verified both in vitro and in vivo at various oxygen concentrations. Ir(III) endoperoxide complex Ir-6 (Figure 7) releases a highly cytotoxic Ir(III) species, an alkoxy radical and 1O2 upon two-photon irradiation in NIR (750 nm), and exhibits nanomolar photocytotoxicity towards hypoxic cancer cells.70
Photoswitching of 1,2-dithienylethene derivatives in Pt-8 (Figure 7) between open and closed forms alters their cytotoxicity.71 The closed form resulting from UVA irradiation is less voluminous and more planar with a greater π conjugation and exhibits stronger DNA binding and subsequent cytotoxicity.
3 Activation Wavelength
3.1 One-photon Excitation (OPE)
OPE is frequently used for light irradiation of photoactivatable metallodrugs and can be achieved by both broadband lamps and lasers. Generally, the choice of activation wavelength in phototherapy is restricted by the absorption spectra of photoactivatable prodrugs and based on a compromise between tissue penetration and photon energy.3c Irradiation at the λmax leads to formation of a populated excited state. The conjugation with coumarin-based ligands red-shifts the absorbance of cyclometalated Ir(III) complexes from 305 nm (Ir-8, Figure 8) to ca. 550 nm (Ir-7, Figure 8), enhancing the photocytotoxicity towards cisplatin-resistant A2780cis cells exposed to green light (520 nm) in both normoxia and hypoxia. In contrast, Ir-9 (Figure 8) without an electron-donating group (EDG) on the coumarin ligand displays a blue-shifted absorbance (436 nm), and has no apparent green-light photocytotoxicity.72 The same strategy has been applied in cyclometalated Ru(II) complex Ru-2 (Figure 4), which kills cancer cells in the presence of light with wavelengths ranging from 620–770 nm.21 Among them, 620 nm gave the lowest IC50 values, but surprisingly, 740 nm irradiation where little light is absorbed also gives good activity. Os(II) coordinated to simple polypyridyl ligands as in Os-4 (Figure 8) exhibit a weak broadband absorbance covering the region up to 720 nm and high one-photon NIR phototoxicity (740 nm), both in vitro and in vivo.73 OPE may cause photobleaching of photocatalysts. Irradiation with 650 nm light induces acute photobleaching (> 60 %) of the Ru(II)-cyanine complex Ru-11 (Figure 8).74 In the excited state, a bond from the fluorophore moiety is broken, reducing its absorption. Notably, diazido Pt(IV) complex Pt-9 (Figure 8) can be photoactivated by green light, although it has no detectable absorbance at 520 nm.46b This suggests that there is a complicated relationship between absorption and activation wavelengths, which requires further exploration.
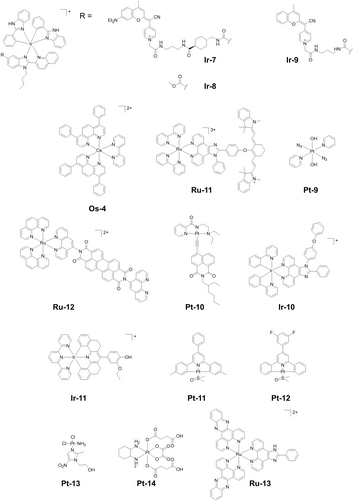
Metallo-prodrugs activated by single-photon, multi-photon, or X-ray irradiation.
OPE is also frequently applied to complexes designed to combat antimicrobial resistance (AMR), a global issue. Photoactivatable metallodrugs for antimicrobial PDT (aPDT) can introduce damage to multiple bacterial targets by ROS generation.75 The Ru(II) drug TLD1433 for example shows activity against Staphylococcus aureus and resistant Staphylococcus aureus under green light through ROS generated by Type I and Type II mechanisms.76 Ru-12 (Figure 8) effectively eradicates Sporothrix brasiliensis and Candida albicans when exposed to green light.77 Ir-3 (Figure 8) also exhibits good antibacterial activity against Streptococcus mutans and Staphylococcus aureus with potent toxicity under visible light irradiation.33
3.2 Multi-Photon Excitation (MPE)
Compared with traditional OPE, MPE can red-shift the activation wavelength to the NIR for deeper tissue penetration.78 Two-photon absorption was proposed by Göeppert-Mayer (Physics Nobel Prize 1963). Two photons are absorbed simultaneously, leading to two-photon excitation (TPE), with each photon contributing half of the required energy.79 As a result, to populate the same excited state as OPE, light at ca. 2× the wavelength is required for TPE.78c The presence of conformational rigidity and an extended conjugated system are usually necessary for a high two-photon absorption cross-section to allow TPE.78b The TPE metal complex dipyridinium Tl(III) pentachloride was reported in 1974, and current mainstream TPE metal anticancer agents are mainly Ru(II), Ir(III) and Pt(II) complexes.78b, 78c Pt-10 (Figure 8) exhibits a substantial TPA cross-section (δ) of 180.64 GM at 825 nm and generates both superoxide and hydroxyl radicals, and induces cell death and tumor destruction upon exposure to NIR two- photon light.80 The TPE coumarin-based complex Pt(IV) oxaliplatin derivative Pt-7 (Figure 5) can be photoactivated using 880 nm laser light. This can oxidize intracellular biomolecules directly and eradicate tumor cells efficiently both in vitro and in vivo upon irradiation in an oxygen-independent way, overcoming the barrier of hypoxia.48c
Different from TPE, three- and four-photon excitations are more frequently used in imaging rather than therapy. Ir-10 (Figure 8) with a Stokes shift>200 nm, is a promising three-photon phosphorescence agent with a low threshold for three-photon excitation power (0.5 mW, 980 nm) to achieve a tissue penetration depth of ca. 450 μm. This allows 3D imaging and reconstruction of brain vasculature in a living specimen.81 Ir-11 (Figure 8) in the aggregated state shows a high three-photon absorption cross-section of 300×10−92 cm6 s2 photon−2, generating ROS through aggregation-induced intersystem crossing, leading to photocytotoxicity under near infrared (820 nm) irradiation.82 Antimicrobial three-photon-activated Pt(II) complex Pt-11 (Figure 8) can destroy bacterial cell walls and damage DNA, speeding up wound healing in mice with 1450 nm irradiation.83 Fluoro-substituted Pt(II) complex Pt-12 (Figure 8) with a similar structure to Pt-11 has triplet metal-to-ligand charge-transfer transitions at ca. 460 nm and undergoes four-photon excitation (FPE) at 1600 nm using a femtosecond laser.84 Strong colocalization in lysosomes in HeLa cells was observed due to its high fluorescence in the presence of lecithin. However, its FPE photocytotoxicity in vitro and in vivo was not investigated.
Despite the promising tissue penetration of MPE, its clinical application is limited by the necessity of high-power femtosecond solid-state lasers and small irradiation volumes. With advance in light devices, MPE therapy might one day become clinically useful.
3.3 X-ray Excitation
Platinum-based anticancer drugs, such as cisplatin and oxaliplatin, have been used as radiosensitizers to potentiate radiotherapy.85 Similar to PDT, radiotherapy suffers from hypoxia resistance.86 Pt(II)-nitroimidazole complexes, e.g. Pt-13 (Figure 8), have been developed as hypoxia-sensitive anticancer radiosensitizers due to the reduction of the nitroimidazole under hypoxia.87 Pt(IV) radiosensitizers have also been reported, usually as nanomaterials.88 Recently, radiation-induced reduction of Pt(IV) complexes, e.g. Pt-14 (Figure 8), to form cytotoxic Pt(II) drugs in water was reported, showing promising tumor regression in mice.89 Ru(II) polypyridyl complexes can act as radiosensitizers, on their own or supported in nanostrucutures.90 Ru-13 (Figure 8) increases DNA damage-marker histone H2AX when exposed to ionizing radiation and decreases cell survival.91 Compared with regular phototherapy, X-ray excitation allows much deeper tissue penetration, but suitable radiosensitizers are still little explored.92
4 Cellular Mechanisms of Action
4.1 Mechanisms of Death
Targeting specific cell death pathways is an emerging drug design strategy since cell death modes affect drug efficacy significantly. Common cell death mechanisms induced by metallodrugs have been systemically reviewed.1b, 1d, 93 Thus, only death mechanisms induced by photoactivatable metallodrugs are discussed here.
ROS-mediated apoptosis is the most common mechanism of death for metal-based photosensitisers.3c Photoreleasable metallodrugs can also induce apoptosis. Diazido Pt(IV) complex Pt-15 (Figure 9) kills HL60 leukemia cells via an apoptotic mechanism.94 However, its death mechanism does not rely only on the activation of the caspase 3 pathway, p53 protein accumulation is not observed in cells treated with Pt-15 and UVA irradiation.46a Apoptosis can also be induced by Pt-9 (Figure 8) and monitored by a fluorescence resonance energy transfer (FRET) pair consisting of a far-red fluorescence donor Cy5 and a NIR quencher Qsy21, linked by a peptide sequence that can be recognized and cleaved by caspase-3.95
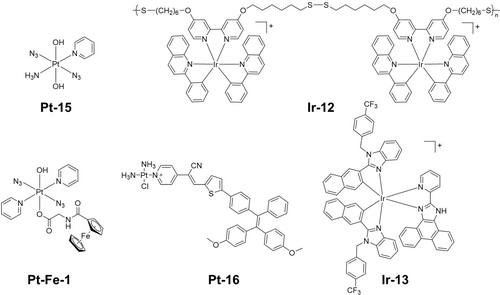
Photoactivatable metal complexes which kill cancer cells by different mechanisms.
Ferroptosis is an iron-dependent programmed cell death regulated by iron homeostasis, lipid metabolism, and the glutathione-dependent redox balance.96 The Fenton reaction in ferroptosis can produce oxygen from hydrogen peroxide for PDT, and PDT produces ROS to deplete GSH thus enhance ferroptosis.97 The biodegradable Ir(III) polymer Ir-12 (Figure 9) can induce ROS-mediated apoptosis via two-photon PDT and ferroptosis by depletion of GSH, resulting in hybrid cell death.98 Pt-Fe-1 (Figure 9) contains axial coordination of a ferrocene carboxylate, with Pt(IV) and Fe(II) 6.07 Å apart, which red-shifts the absorption spectrum of the parent Pt(IV) complex.99 Ferrocene behaves as a light antenna, allowing charge transfer from iron to platinum, then photoactivation and photocytotoxicity with green light under both normoxia and hypoxia. Ferroptosis was the major mechanism of cell death induced by Pt-Fe-1 upon irradiation.
Necroptosis is a programmed form of necrosis, dependent on a receptor-interacting protein kinase (RIPK-1 and RIPK-3), mixed linkage kinase domain-like protein (MLKL) and Z-DNA binding protein 1 (ZBP1).100 An aggregation-induced emission (AIE)-based monofunctional Pt-16 (Figure 9) enters cells via the endocytosis pathway and locates mainly in lysosomes, and then inhibits tumor cell growth that can be suppressed by ferroptosis or necroptosis inhibitors.101
Metal-based photosensitizers can induce ROS-mediated endoplasmic reticulum (ER) stress that can initiate immunogenic cell death (ICD), in which an adaptive immune response is activated to kill cancer cells.102 The biochemical hallmarks of ICD include (i) translocation of ER-resident calreticulin (endo-CRT) to the cell surface (ecto-CRT) in early apoptosis; (ii) active secretion of ATP; (iii) extracellular secretion of nuclear high-mobility group box 1 protein (HMGB1) in late apoptosis.1b, 103 Ir-13 (Figure 9) partially localizes in the ER and generates ROS upon irradiation, thus inducing oxidative damage and ICD processes.104 Except for apoptosis, Pt-9 also kills cancer cells via ICD. Cells treated with Pt-9 and light irradiation exhibit the three ICD hallmarks, and autophagy plays a key role in the mechanism.105
4.2 Cellular Targeting
The main cellular targets of photoactivatable metallodrugs include nuclei, mitochondria, lysosomes, and the endoplasmic reticulum.
Nuclei are among the most targeted cellular organelles for phototherapeutic agents, since they are responsible for controlling all cell biology at the transcriptome level.106 However, drugs that target only the nuclei can potentially cause DNA mutations which might discourage their continuous use.107 Photocatalysts can interact with DNA via intercalative or non-intercalative modes,108 and oxidize guanine directly,109 or indirectly causing damage through ROS generation.110 Dinuclear polypyridyl Ru(II) complexes are DNA probes with high affinity (> 107 L/mol), which enter the nuclei of live cells and stain chromatin.111 Ru(II) complex Ru-14 (Figure 10) bearing a dppz-type ligand with DNA intercalation ability shows high nuclear accumulation of > 40 %, and thus high photocytotoxicity towards Hela cells under blue light.112 DNA is important target for current clinical Pt drugs but only ca. 1 % cisplatin enters nuclei.113 In contrast, photoactive diazido Pt(IV) complexes can show high accumulation in nuclei, especially after irradiation.99, 114 Metallodrugs can accumulate in organelles in the cytoplasm in the dark, then escape upon irradiation due to the generation of ROS, then enter nuclei and kill cancer cells.115
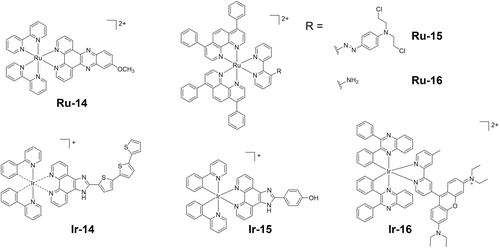
Photoactivatable metal complexes which target cellular organelles. These complexes all have chiral metal centers. The biological properties of the separated enantiomers Ir-14Δ and Ir-14Λ differ (ref. 118).
Mitochondria play a pivotal role in energy production and regulate several cell death pathways (apoptosis, necrosis, and autophagy) and hence are important targets for photoactivatable metallodrugs.116 Lipophilic and cationic metallodrugs can enter mitochondria due to the negative potential across the outer and inner membranes. The strong electron-withdrawing azo group quenches the emission of Ru-15 (Figure 10). This bond can be reduced in hypoxic cells to release the free aniline mustard (NM) and the photocatalytic and emissive polypyridyl Ru(II) complex Ru-16 (Figure 10), which accumulates in the mitochondria and exerts high cytotoxicity towards HepG2 cells.117 Enantiomers Ir-14Δ and Ir-14Λ (Figure 10), the Ir(III) analogues of TLD1433, both locate in mitochondria and potentially lead to apoptotic cell death with the generation of 1O2.118 Notably, Ir-14Λ displays higher photocytotoxicity than its Δ isomer, indicating the need to consider the possible influence of the chirality of such octahedral metal complexes on drug activity.
The acidic environment of lysosomes (lumen pH ca. 4.5–5) allows them to digest biomolecules from autophagy and endocytosis.106, 119 Thus, lysosome-targeting drugs are usually pH-dependent and rely on the disruption of lysosomal membrane integrity.119, 120 Ir-15 (Figure 10) accumulates in lysosomes, where it is protonated and exhibits increased phosphorescence, enhanced 1O2 photo-generation and NADH photooxidation, therefore promising for in vitro and in vivo photocytotoxicity.120b
ER stress is generated by the accumulation of misfolded or unfolded proteins, inducing programmed cell death.121 Ir-16 (Figure 10) shows highly specific ER localization and photo-induced ROS generation, and apoptotic cell death upon irradiation.122 Notably, ROS-mediated ER stress can cause ICD as discussed in section 4.1.
5 Metallomics—Excited State Species and Photoproducts
Metallomics explores the speciation and quantitative distribution in cells and tissues of metal ions and their correlations with genomes and proteomes, thus providing a valuable tool for the understanding of mechanisms of action of metallodrugs and their therapeutic activities.123 The identification of excited state species, photoproducts, and their target biomolecules is of critical importance in the clinical development of photoactivatable metallodrugs.
5.1 Excited State Species
Photocatalysts form transient 3MLCT triplet states upon irradiation, momentary charge-separated states with the metal being oxidized and the ligand reduced.124 The photocatalyst can return to its ground state through a photocatalytic process with reduced photobleaching facilitated by interaction with oxygen, leading to the formation of singlet oxygen (Type II) or other ROS (Type I). Time-resolved spectroscopies such as the lifetime of decay from the excited state, and flash photolysis/transient absorption give further insights into the quenching process. This determines electron density movement after irradiation and can aid understanding of the photocatalytic process.124, 125 However, the full experimental characterization of excited states is challenging due to reasons including the small energy range of numerous excited states, their short lifetimes, forbidden electronic transitions and spectroscopic dark states.126 In contrast, the electronic and geometrical features of excited-states, and the energy gap between excited states and ground states can be described by DFT and TD-DFT calculations. Hence DFT can provide valuable insights into the photophysics and photochemistry of photoactivatable metallodrugs and contribute to understanding their mechanisms of action.126, 127
Ru(II), Os(II) and Ir(III) complexes have their HOMOs largely composed of metal orbitals (dπ), while the LUMOs mainly consist of the ligand π* orbitals.128 Thus, in the excited state the metal is virtually oxidized while the ligand is reduced in a charge separation state through a MLCT transition. If the 3IL*/3LLCT* transition is lower in energy compared to the 3MLCT*, this state can be achieved with a longer lifetime compared to the 3MLCT* state.129 Modifications of the ligands can modify the HOMO and LUMO energies. Electron-withdrawing substituents on ligands can remove electron density from the metal and destabilize the π*-orbitals, while electron-donating substituents can stabilize the transient charge separation state (Ru(III)/(ligand)⋅−) to reduce the energy gap.130 Both methods can red-shift the MLCT transitions. A DFT-guided search on Ru(II) photosensitizers Ru-17 to Ru-23 has been used to maximize the therapeutic window and favor singlet oxygen formation (Figure 11a). The energy gap between HOMO and LUMO was 3.99 eV when no group was present and reduced to 3.17 eV with a strong electron-donating group.131 Notably, except for the effect on the absorption maximum (λmax), the energy gap affects the lifetime, quantum yields of emission and singlet oxygen formation. Ru-23 with the smallest energy gap showed the largest λmax, lowest emission lifetime and quantum yields, as well as singlet oxygen generation in acetonitrile. Interestingly, despite having the lowest singlet oxygen generation in PBS, Ru-22 displays high photocytotoxicity with light exposure at 595 nm by disturbance of mitochondrial respiration and glycolysis.
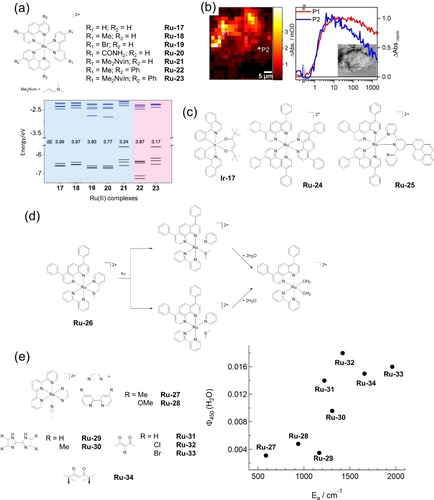
(a) Photocatalytical complexes Ru-17−Ru-23 and their computed frontier orbital energies and HOMO–LUMO gaps (in eV). (b) Distribution of TLD1433 in MCF7 cells obtained by spatially-resolved TA microscopy and two normalized TA kinetic traces. (c) Photocatalysts which oxidize biomolecules upon irradiation. (d) Proposed activation pathways of Ru-26 involving 3MC states as intermediates for the formation of bisaquated photoproducts. (e) Relationship between the ligand exchange quantum yield in water (Φ450) and the activation energy from the 3MLCT to the 3MC state (Ea) in Ru-27−Ru-34. Parts (a), (b) and (e) are reproduced from ref. 131, 132, 144a, respectively, with permission.
The excited state dynamics of TLD1433 (Figure 1) in MCF7 breast cancer cells using a modified transient absorption (TA) system in a vertical position that orients the laser pulses perpendicular to the plane of the optical table, has been studied.132 TLD1433 (40 μM) in fixed MCF7 cells showed bi-exponential kinetics related with vibrational conversion from 3MLCT to 3ILCT (τ = 1.5 ps) and vibration relaxation within 3ILCT (13 ps). In cellulo measurements showed no decay of the long-lived 3ILCT in a 1.9 ns window, while a decay (35 %) was observed in aqueous solution. The same kinetic profile was observed for highly concentrated TLD1433 in water (500 μM) or when TLD1433 interacts with calf thymus DNA (ct-DNA) in solution. Moreover, TA microscopy showed the heterogeneity in excited-state dynamics across different cellular regions where the photosensitizer was densely concentrated, reflecting local environmental influences (Figure 11b). This is a good example of how the cellular medium may alter the lifetime of the excited states, making them different from those tracked in solution. This work opens the possibility to explore in depth excited-state dynamics of photosensitizers in their relevant biological environment.
Singlet oxygen is an important active species generated by photocatalysts and associated closely with metallodrugs in excited states. A high quantum yield for intersystem crossing from 1MLCT* to 3MLCT*/3IL* results in an effective Type II energy transfer to molecular oxygen. The formation of singlet oxygen depends on the interaction or collision between these triplet excited states with 3O2. Two oxygen excited states can be reached: 1Δg at 22 kcal mol−1 (0.954 eV) above the ground state with paired electrons in oxygen's degenerate π antibonding orbitals, and 1Σg at 37 kcal mol−1 (1.62 eV) above the 3O2 ground state which quickly decays to 1Δg. The 1Δg state is long-lived (emission at 1270 m) and is responsible for singlet oxygen activity. The energy transfer between 3MLCT*/3IL* and 3O2 is effective because both the complex and oxygen share the same multiplicity, and the metal excited state has a slightly higher energy.133 If the excited state of the photocatalyst is too high in energy, it will be poorly quenched by oxygen, resulting in low oxygen sensitization.134 Tracking singlet oxygen inside cells is challenging, especially given its short lifetime (ca. 3.5 μs) and rapid, localized reactivity, as it is readily deactivated or undergoes oxidative bimolecular reactions with cellular substrates.135
The combination of advanced chemical techniques aligned with bioinformatics and proteomics provides a new perspective on exploring selective oxidation of substrates within the cells. When irradiated with blue light (465 nm), Ir-17 (Figure 11c) induces specific oxidative attack on cancer cell (A549) proteins by forming oxo-adducts of histidine residues in aldose reductase and heat-shock protein-70 confirmed by LC-MS/MS. Moreover, this localized attack by singlet oxygen generated a cascade reaction inside the cell, leading to an up-regulation of proteins involved in the glycolytic pathway.15a The activity of photosensitizers which target the nuclei may involve oxidation of DNA or merely photocleavage. In an in vitro model, irradiation of ct-DNA in the presence of Ru-24 (Figure 11c) led to the formation of the oxidized and mutagenic product 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG).136 Bioinformatic analysis of expressed proteins in A549 cells after exposure to Ru-25 (Figure 11c) under blue light (420 nm) suggested oxidative DNA damage, as indicated by the inhibited expression of DNA repair-related proteins such as RBX1 and GPS1.137
Photostability is regarded as an essential property of PDT photoactivatable metallodrugs. Recently photosubstitution has demonstrated potential for controlled light activation, with promising application in PACT anticancer agents.38b Compared with photosensitizers relying on MLCT states, dissociative 3MC states from 3MLCT states that lie close enough in energy are favorable for photosubstitution of polypyridyl Ru(II) and other d6 metals complexes, e.g. Ir(III) and Re(I).38b, 38c For PACT, once the thermal barrier to cross from 3MLCT to 3MC is overcome, an electron from LUMO is promoted to an antibonding metal-ligand orbital, altering the geometry in the excited state and lengthening the Ru-photolabile ligand bond.138
Various approaches to overcome the thermal barrier have been used: sterically hindering ligands (lower 3MC energy),139 substituents on polypyridyl ligands (rise 3MLCT energy),140 and coordination of photoreleasable monodentate ligands.141 In all these cases, the photochemotherapeutic effect of PACT is achieved without direct oxygen involvement; if ROS are involved, they are produced via a type I mechanism. The excited state of PACT prodrugs competes between photosubstitution, light emission, and 1O2 generation, the latter two pathways being inefficient.51
The presence of the 2-(methylthiomethyl)pyridine (mtmp) ligand in Ru-26 stabilizes the vacant molecular orbitals responsible for the generation of the 3MC states, facilitating its population from a 3MLCT state, thus promoting the photo-induced hydrolysis of the complex (Figure 11d).142 Significant inhibition in the growth of conjunctival melanoma growth in a zebrafish orthotopic xenograft model was observed on treatment with Ru-26 and green light (520 nm).143 Ligand dissociation can not only take place from 3MC states, but also directly from the 3MLCT state as evidenced both experimentally and theoretically.144 A series of complexes based on [Ru(tpy)(L)(CH3CN)]n+ Ru-27 to Ru-34 undergo CH3CN ligand dissociation upon irradiation, and their ligand-dissociation quantum yield increases as the activation energy, determined by Arrhenius analysis, for population of the 3MC state from the 3MLCT state increases (Figure 11e), which is counterintuitive and indicates ligand dissociation directly from the 3MLCT state.144a
Highly dissociative triplet states of Pt-9 (Figure 8) involving the N3 ligands have been identified by DFT calculations with the Pt−N3 distances significantly elongated by 0.5 Å (Pt−N1) and 0.6 Å (Pt−N2) compared to the ground-state geometry, resulting in the formation of the [PtII(OH)2(py)2] photoreduced product (Figure 12a).145 However, although photosustituted product [PtIV(N3)(OH)3(py)2] was detectable by LC–MS after irradiation, calculations indicate that the substitution takes place from Pt-9 in its singlet ground state, a difference from experiment which requires further investigation. DFT calculations show that conjugation of Fe(II) or Ir(III) to Pt(IV) in Pt-Fe-1 and Pt-Ir-1, respectively, gives rise to metal-metal charge-transfer from Fe(II) or Ir(III) to Pt(IV) via a long flexible bridge, and explains their accelerated photoactivation rates and red-shifted photoactivation wavelengths (Figure 12b and c).99, 114
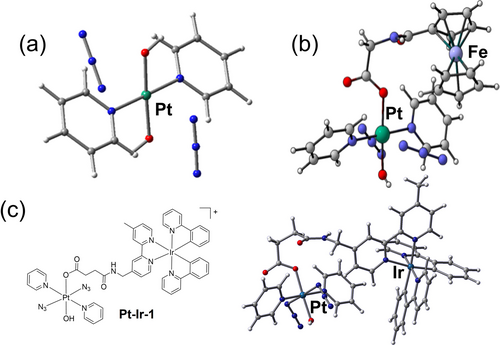
Optimized structures of (a) Pt-9 (see Figure 8) and (b) Pt-Fe-1 (see Figure 9), in excited triplet states showing dissociative character, which leads to the release of azidyl radicals. (c) The chemical structure of ground state and optimized structure of an excited triplet state of Pt-Ir-1. Parts (a), (b) and (c) are reproduced from ref. 145, 99, 114, respectively, with permission.
5.2 Photoproducts
Photoactivated species and photoproducts from reactions with small biomolecules in solution or cell extracts can be analyzed by a variety of techniques. For example, Pt-9 has been investigated by vibrational spectroscopy, attenuated total reflection Fourier transform infrared (ATR-FTIR) and Raman, and synchrotron radiation far-IR spectroscopies, and its photoactivation monitored by UV/Vis, ATR-FTIR, LC-MS and LC-ICP-MS.46b, 145, 146 The combination of different analyses allows a more comprehensive understanding of the photoactivation mechanism of Pt-9, which undergoes not only photoreduction but also photosubstitution. Its reactions with 5’-GMP under different light exposures were also monitored by 1H and 195Pt NMR, LC-MS and ATR-FTIR.46b, 146b Both mono- and bi-GMP Pt adducts were identified in the irradiated solutions.
The cellular tracking of Ru(II), Os(II) and Ir(III) photocatalysts using luminescence microscopy, such as confocal microscopy,13a, 73, 147 two/multi-photon imaging,62b, 148 structured illumination microscopy (SIM),149 and (two-photon) phosphorescence lifetime imaging ((TP)PLIM),150 is achievable since they are often phosphorescent. Confocal microscopy has revealed the endocytic cellular uptake and localization in Golgi and lysosomes of Ru-35 in HeLa cells, and morphological changes of HeLa cells treated with Ru-35 and light (Figure 13a).151 Compared with one-photon microscopy (OPM), two/multiple-photon microscopy (T/MPM) uses more penetrating low-energy photons, allowing imaging of subcellular features hundreds of micrometers deep into the sample. The design of two-photon-absorbing photocatalysts is crucial. The imaging obtained with Ir-18 and OPM is poor, whereas TPM (λex=750 nm) allows imaging in A375 spheroids for 200 μm in depth (Figure 13b).152 The super resolution (SR)-SIM images indicate mitochondrial accumulation of Ru-36 and its binding to mt-DNA (Figure 13c).153 Notably, SR-SIM shows much higher luminescence intensity for the Δ-enantiomer in the nuclei and mitochondria than the Λ-enantiomer. The luminescence and emission lifetime of lysosomal-targeted Ir-19 are pH-dependent, varying from 463 to 27 ns in cells between pH 4 to pH 8, respectively (Figure 13d).154 The degree of lysosomal damage induced by Ir-19 was monitored by TPPLIM. The 48 ns lifetime after irradiation (808 nm) suggested that lysosomal membrane damage results from the release of proteases with concomitant increase in the organelle pH.
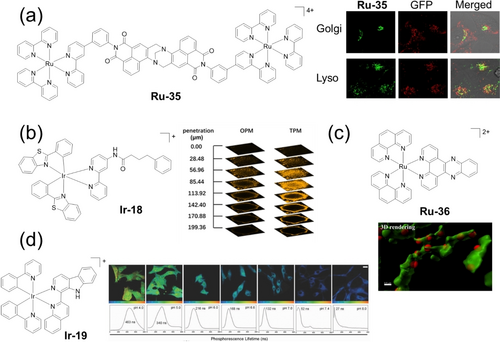
(a) Confocal microscopy of Hela cells showing colocalization of Ru-35 with lysosomes and the Golgi apparatus. (b) One-(OPM) and two-photon (TPM) excited z-stack confocal laser scanning microscopy of A375 spheroids upon incubation with Ir-18 for 12 h. (c) 3D SIM images of HeLa cells treated with Ru-36 (red) and MTG (green). (d) TP-PLIM images of U87 cells incubated with Ir-19 at different pH values. Parts (a), (b), (c) and (d) are reproduced from ref. 151-154, respectively, with permission.
Synchrotron radiation covers a wide range of the electromagnetic spectrum, providing for example XRF, X-ray absorption spectroscopy (XAS), X-ray tomography (XRT) and infrared microspectroscopy with near-native state imaging of biological samples with high resolution and deep penetration.155 Diazido Pt(IV) complex Pt-19 (Figure 14) with an axial coumarin ligand exhibits remarkable photo-enhanced cellular Pt accumulation and photo-induced cellular damage with extreme alterations to multiple cellular components in PC3 prostate cancer cells, including formation of vacuoles, as visualized by cryo soft X-ray tomography (cryo-SXT).156 Nano-XRF shows enhanced cellular accumulation of Pt from Pt-19 in PC3 cells upon irradiation (Figure 14).156b XANES analysis suggested only partial reduction of the prodrug, indicating that photoexcited Pt(IV) species might also exert phototoxicity as well as the Pt(II) photoproducts. Nano-XRF analysis of A549 cells treated with Pt-Ir-1 (Figure 12c) indicated the cleavage of this dinuclear complex in the dark and significantly enhanced Pt accumulation upon irradiation, especially in the nuclei.114 The presence of Ir(III) promotes the photoreduction of Pt(IV) within cells as monitored by XANES. These studies illustrate the importance of studying the in-cell chemistry of metallodrugs rather than merely model reactions.
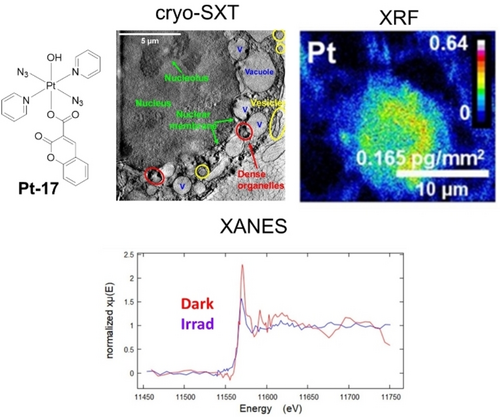
Cryo-SXT, XRF and XANES images of PC3 human prostate cancer cells treated with Pt-17, exhibiting the damaged organelles, enhanced cellular Pt accumulation and photoreduction of Pt(IV). The Figure is reproduced from ref. 156b with permission.
6 Summary and Outlook
The present landscape of photoactivatable metallodrugs is characterized by significant advances in their design and medicinal use. Several compounds have now reached clinical trials and gained regulatory approval. Light-activated drugs have emerged as effective and targeted cancer therapies by selective activation through appropriate light exposure, which allows precise control in space, time and dose, reducing off-target effects and systemic toxicity.
One key area of progress is the development of photosensitizers that can overcome the limitations of traditional PDT, particularly in hypoxic tumor environments by generating ROS independently of oxygen. PACT metallodrugs are being developed to overcome this limitation of PDT. Novel PDT agents that work under a Type I mechanism, such as TLD1433 (in Phase II clinical trials) are also being explored.
Currently, the use of quantum calculations and fluorophores can assist the design and ligand choice to render metallodrugs with red-shifted activation wavelengths, enhancing tissue penetration and minimizing damage to healthy tissues, mainly for one-photon activation. Future developments are likely to focus on multi-photon excitation techniques, such as two-photon and even three-photon excitation. This will allow deeper tissue penetration using near-infrared (NIR) light, which is less harmful to healthy cells and ideal for non-invasive treatments. Although more targeted, the challenge is to develop multiphoton beams to cover a significant area of treatment in a reasonable time. More efficient two-photon-active metal complexes and improved design of clinical light sources for their activation are needed.
Future research is also likely to focus on enhancing the tumor-targeting abilities of photoactivatable metallodrugs. By using this strategy, more effective delivery and tumor penetration can be obtained in combination with red-shifted absorption. Conjugation with tumor-specific antibodies and peptides is allowing development of dual-responsive systems, e.g. pH and light-activated, and may contribute to the next generation of photoactivatable drugs.
Another promising direction involves the combination of phototherapy with other treatment modalities, such as immunotherapy or chemotherapy, for synergistic effects. Metallodrugs can potentially induce ICD, activating the immune system to target cancer cells, an area that could dramatically expand their therapeutic impact.
Despite the promising outlook and the clinical translation of these metallodrugs, there are significant challenges to overcome. While several metal-based agents, like TLD1433, have entered clinical trials, broader clinical adoption will require further advances in controlling the pharmacodynamics and biodistribution of metallodrugs that are highly stable in the dark but readily activated by light. This strategy can lead to an optimization of the balance between the dark and light-activated species, which will ultimately address potential long-term toxicity concerns related to metal accumulation, so accelerating drug discovery by improving the safety of metal-based drugs.
Moreover, understanding the precise mechanisms of action and optimizing these drugs for specific cancer types will be essential for their success in clinical settings. The use of advanced imaging techniques, such as synchrotron XRF and cryoXST, cryoSIM, SIMS, LA-ICP-MS, SEM-EDXS, and confocal microscopy, will continue to be crucial for tracking the localization and activation of metallodrugs in real-time. Tackling the challenges of identifying excited state pharmacophores using recently developed metallomics procedures is still in its infancy.
The future of photoactivatable metallodrugs is highly promising, with ongoing research likely to yield more effective and targeted therapies. Enhanced tissue penetration, multi-photon activation, and integration with other treatment strategies will shape their development, potentially transforming the management of difficult-to-treat cancers. As research progresses, we can expect to see increasingly sophisticated and effective compounds entering clinical trials and eventually reaching patients. The unique properties of metal complexes, combined with the precise control offered by light activation, position these drugs at the forefront of next-generation cancer treatments and beyond. However, addressing clinical challenges such as drug stability, activation control, and comprehensive mechanistic understanding will be key to realizing their full potential as a contribution towards revolutionizing targeted therapies.
Acknowledgments
We thank the Engineering and Physical Sciences Research Council (EPSRC grant no. EP/P030572/1), Diamond Light Source Ltd (DLS, Oxfordshire), Anglo American Platinum, and the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 945380 (for RCM) for funding.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Biographical Information
Dr. Huayun Shi obtained her PhD degree with a Chancellor's International Scholarship in the Department of Chemistry at University of Warwick (UK) under supervision of Prof. Peter Sadler in 2019, then continued her research in Sadler group as a Post-doc from 2020 sponsored by ICURe, MRC-IAA and Anglo-American. She is now an Assistant Professor in the School of Public Health at Xiamen University. Her research focuses on the development of novel photoactive platinum complexes with azido ligands in cancer therapy and their mechanisms of action.
Biographical Information
Dr. Rafael C. Marchi gained his PhD in Chemistry with distinction from the Federal University of São Carlos in 2021. In 2022, he joined the University of Warwick as a Marie Sklodowska Curie EUTOPIA-SIF COFUND Postdoctoral Fellow under the mentorship of Prof. Peter Sadler, with further training at the University of Ljubljana (Slovenia) with Prof. Iztok Turel and University of Reading with Prof. Christine Cardin. His research focuses on photoactive cell redox modulators based on Mg(II), Ru(II), and Ga(III) with anticancer and antimicrobial activities.
Biographical Information
Peter Sadler obtained his BA, MA and DPhil at the University of Oxford. Subsequently he was a Medical Research Council Research Fellow at the University of Cambridge and National Institute of Medical Research. From 1973–96 he was Lecturer, Reader and Professor at Birkbeck College, University of London, and from 1996–2007 Crum Brown Chair of Chemistry at the University of Edinburgh. In 2007 he took up a Chair in Chemistry at the University of Warwick as Head of Department, where he is now a Professor.







