The Role of Electron Transfer in Copper-Mediated C(sp2)−H Trifluoromethylation
Abstract
We report copper(II) and copper(III) trifluoromethyl complexes supported by a pyridinedicarboxamide ligand (L) as a platform for investigating the role of electron transfer in C(sp2)−H trifluoromethylation. While the copper(II) trifluoromethyl complex is unreactive towards (hetero)arenes, the formal copper(III) trifluoromethyl complex performs C(sp2)−H trifluoromethylation of a wide range of (hetero)arenes. Mechanistic studies using the copper(III) trifluoromethyl complex suggest that the mechanism of arene trifluoromethylation is substrate-dependent. When the thermodynamic driving force for electron transfer is high, the reaction proceeds through a previously unidentified single electron transfer (SET) mechanism, where an initial electron transfer occurs between the substrate and oxidant prior to CF3 group transfer. Otherwise, a CF3 radical release/electrophilic aromatic substitution (SEAr) mechanism is followed. These studies provide valuable insights into the role of strong oxidants and potential mechanistic dichotomy in Cu-mediated C(sp2)−H trifluoromethylation.
Introduction
The incorporation of the trifluoromethyl group (CF3) into organic compounds plays a key role in the development of new materials, pharmaceuticals, and agrochemicals.1-4 Copper-based catalysts have been widely employed for the catalytic formation of C−CF3 bonds.5-7 For example, McLoughlin et al. reported the Cu-catalyzed cross-coupling of iodofluoroalkanes and iodoarenes in 1969.8 Since this pioneering discovery, the Ar−CF3 cross-coupling reaction has been developed into a reliable synthetic method.9-12 In recent decades, considerable attention has been directed towards the trifluoromethylation of C−H bonds. This has led to the development of copper-catalyzed C(sp2)−H and C(sp3)−H trifluoromethylation methods utilizing various nucleophilic or electrophilic CF3 sources, e.g., TMSCF3, Togni's reagents, and Umemoto's regents.10, 13-21
High-valent CuIII−CF3 species have been widely proposed as intermediates in copper-catalyzed C−H trifluoromethylation, which are typically thought to facilitate C−CF3 bond formation via reductive elimination (Figure 1A)14, 15, 20, 22-25 or CF3 radical release/electrophilic aromatic substitution (SEAr) (Figure 1B).11, 26, 27 Studies by Shen24, 28 and Liu20 have provided compelling evidence for the reductive elimination mechanism, demonstrating C(sp2)−CF3 or C(sp3)−CF3 formation from discrete CuIII−CF3 aryl/alkyl complexes (Figure 1A). Menjon,27 Zhang,29 and others11 proposed an alternative radical pathway, where photolysis or thermolysis of CuIII−CF3 complexes liberates CF3 radical, which can undergo SEAr reaction to furnish the desired trifluoromethylated (hetero)arenes (Figure 1B).29 This mechanism bears resemblance to those followed by other CF3 radical precursors, e.g., CF3SO2Cl, CF3SO2Na, and AgCF3.30-34
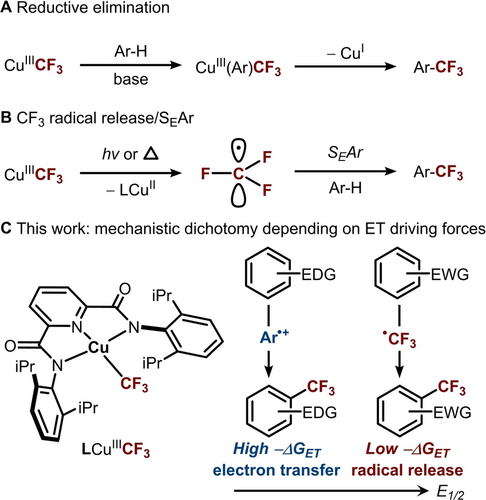
(A−B) Two previously reported mechanisms for C(sp2)−H trifluoromethylation via copper(III) trifluoromethyl complexes. (C) Substrate-dependent mechanism crossover as a function of redox potential from CF3 radical release/SEAr to SET.
Interestingly, regardless of the proposed mechanism, strong chemical oxidants (K2S2O8, Ag2CO3, Cu/O2) or photochemical oxidants, including Ir and Ru photocatalysts, are often employed to facilitate C−CF3 bond formation.34-36 Even in the case of discrete CuIII−CF3 complexes, which are capable of performing trifluoromethylation alone, the inclusion of a co-oxidant markedly enhances the efficiency of the trifluoromethylation.29 These observations suggest that single electron transfer (SET) may play a role in trifluoromethylation. However, this possibility remains unexplored.
Herein, we investigated the role of SET in CF3 transfer from a formal CuIII−CF3 complex to (hetero)arenes. We employed the versatile pyridinedicarboxamide (L) ligand system, a platform that can effectively model organometallic reactions involving copper(II/III) intermediates.37-45 Both the copper(II) trifluoromethyl complex [TBA]LCuIICF3 and formally copper(III) trifluoromethyl complex LCuIIICF3 were synthesized. While [TBA]LCuIICF3 displays minimal C−H trifluoromethylation reactivity, LCuIIICF3 can transfer its CF3 group to a wide range of (hetero)arenes in the presence of oxidant in 42–76 % yields. Mechanistic investigations, including a Marcus study and a radical trap experiment, provide compelling evidence for a substrate-based mechanism crossover from CF3 radical release/SEAr to a previously unidentified SET mechanism.
Results and Discussion
Synthesis of [TBA]LCuIICF3 and LCuIIICF3
Treatment of previously reported [TBA]LCuIIF complex43 with 1.5 equivalents of trimethyl(trifluoromethyl)silane (TMSCF3) in tetrahydrofuran at room temperature for 30 minutes resulted in the formation of a red copper (II) trifluoromethyl complex [TBA]LCuIICF3 complex in 90 % yield (λ=515 nm, ϵ=615 M−1 cm−1 in acetonitrile, Scheme 1). X-ray diffraction (XRD) analysis of [TBA]LCuIICF3 single crystals grown from a pentane/dichloromethane mixture shows a square planar Cu center with an average Cu−N bond length of 2.01(4) Å and a Cu−CF3 bond length of 1.971(3) Å (Figure 2A). Electron paramagnetic resonance (EPR) analysis of [TBA]LCuIICF3 in 3 : 1 toluene:acetone mixture at 30 K shows a rhombic S=1/2 signal with gx = 2.024, gy=2.034, gz = 2.153, and a coupling constant Az = 480 Hz (Figure S57). The cyclic voltammogram of [TBA]LCuIICF3 in acetonitrile with 0.1 M TBAClO4 electrolyte exhibits a quasi-reversible redox couple at E1/2=−0.15 V vs. Fc/Fc+ in 1,2-dichloroethane (DCE) (Figure S2).
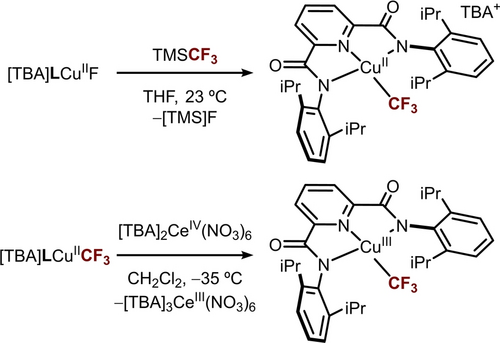
Synthesis of copper(II) and copper(III)−CF3 complexes.
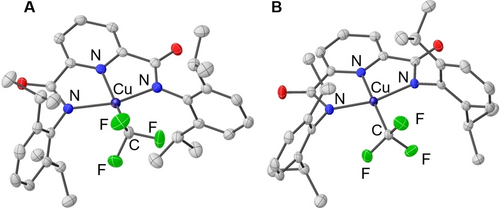
Single crystal XRD structures of (A) [TBA]LCuIICF3 and (B) LCuIIICF3 with thermal ellipsoids shown at a 30 % level of probability. All hydrogen atoms, solvent molecules, and countercations are omitted for clarity. Average C−N bond lengths (Å): [TBA]LCuIICF3 2.01(4), LCuIIICF3: 1.90(4). C−CF3 bond lengths (Å): [TBA]LCuIICF3: 1.971(3), LCuIIICF3: 1.9524(17).46
Treatment of [TBA]LCuIICF3 with oxidant [TBA]2CeIV(NO3)6 (E1/2=0.472 V vs. Fc/Fc+ in DCE) in dichloromethane affords a dark purple copper(III) trifluoromethyl complex LCuIIICF3 in quantitative yield (λ=540 nm, ϵ=10900 M−1cm−1 in acetonitrile, Scheme 1). The isolated LCuIIICF3 is stable at room temperature in a wide range of solvents, e.g., benzene, pentane, acetonitrile, DCE, and dichloromethane. XRD analysis of the single crystals of LCuIIICF3 grown from pentane shows a square planar Cu environment with an average Cu−N bond length of 1.90(4) Å and a Cu−CF3 bond length of 1.9524(17) Å (Figure 2B). Upon oxidation, the Cu−N bonds contract significantly from 2.01(4) to 1.90(4) Å, while the Cu−CF3 bond length remains roughly the same (CuII: 1.971(3) to CuIII: 1.9524(17) Å). 1H NMR in CD2Cl2 shows six sharp resonances within the 0–9 ppm range, accounting for ligand protons and suggesting a diamagnetic ground state for LCuIIICF3 (Figure S35A). 19F NMR analysis in CD2Cl2 shows a single resonance at −41.42 ppm (Figure S35B). Previous studies on formal Cu(III)−CF3 complexes suggest that the Cu−CF3 bond might be quite weak.27 Therefore, we evaluated the Cu−CF3 bond strength in LCuIIICF3 and compared it to (Phen)CuIII(CF3)3 reported by Zhang et al..29 Both complexes exhibit Cu−CF3 bond strengths around 34 kcal/mol (see Supporting Information). The relatively weak Cu−CF3 bond suggests LCuIIICF3 could be utilized for CF3 group transfer.
C(sp2)−H Trifluoromethylation Reactivity of LCuIIICF3
The trifluoromethylation reactivity of complexes [TBA]LCuIICF3 and LCuIIICF3 was then explored. While [TBA]LCuIICF3 is unreactive towards (hetero)arenes, treatment of LCuIIICF3 with 50 equivalents of mesitylene and one equivalent of oxidant ([TBA]2CeIV(NO3)6) in acetonitrile affords the trifluoromethylated product in 72 % yield after 5 minutes at 85 °C (Table 1, entry 1). Without molecular sieves, the yield of the reaction drops to 62 % (Table 1, entry 2), perhaps due to the residual water introduced by [TBA]2CeIV(NO3)6.
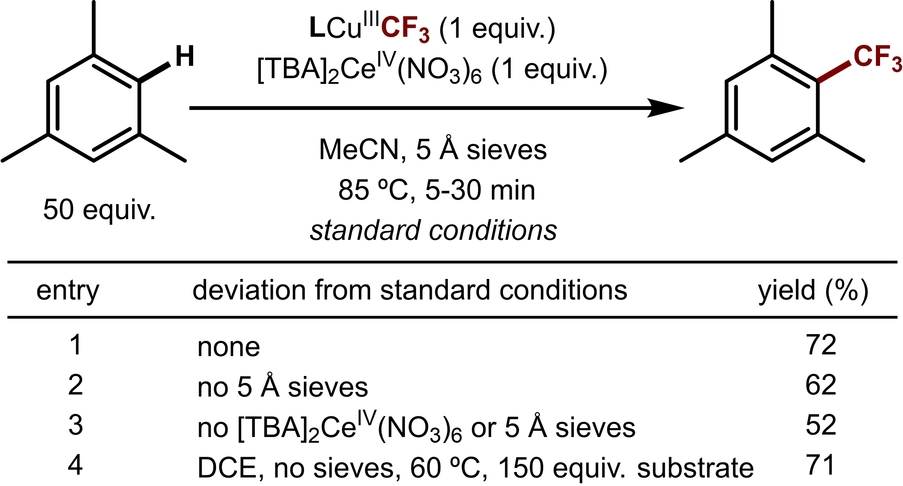
It has been shown previously that the inclusion of a co-oxidant in copper-catalyzed C−H trifluoromethylation can improve the reaction yield significantly.9-15 Zhang et al. attributed these improvements to the SEAr mechanism of the reaction,29 noting that the rearomatization of the arene CF3 radical intermediate requires one equivalent of oxidant (Scheme 2). Similarly, we found that the inclusion of one equivalent of oxidant increases the yield from 52 % (without oxidant, Table 1, entry 3) to 72 % (with oxidant). Interestingly, while the reaction with mesitylene requires 30 minutes to complete without oxidant, it reaches completion in only 5 minutes when oxidant is included, as indicated by the disappearance of the dark purple color of LCuIIICF3. This observation implicates the involvement of the oxidant in the rate-determining step.
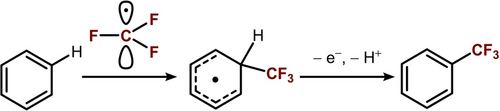
Proposed SEAr mechanism for C(sp2)−H trifluoromethylation.
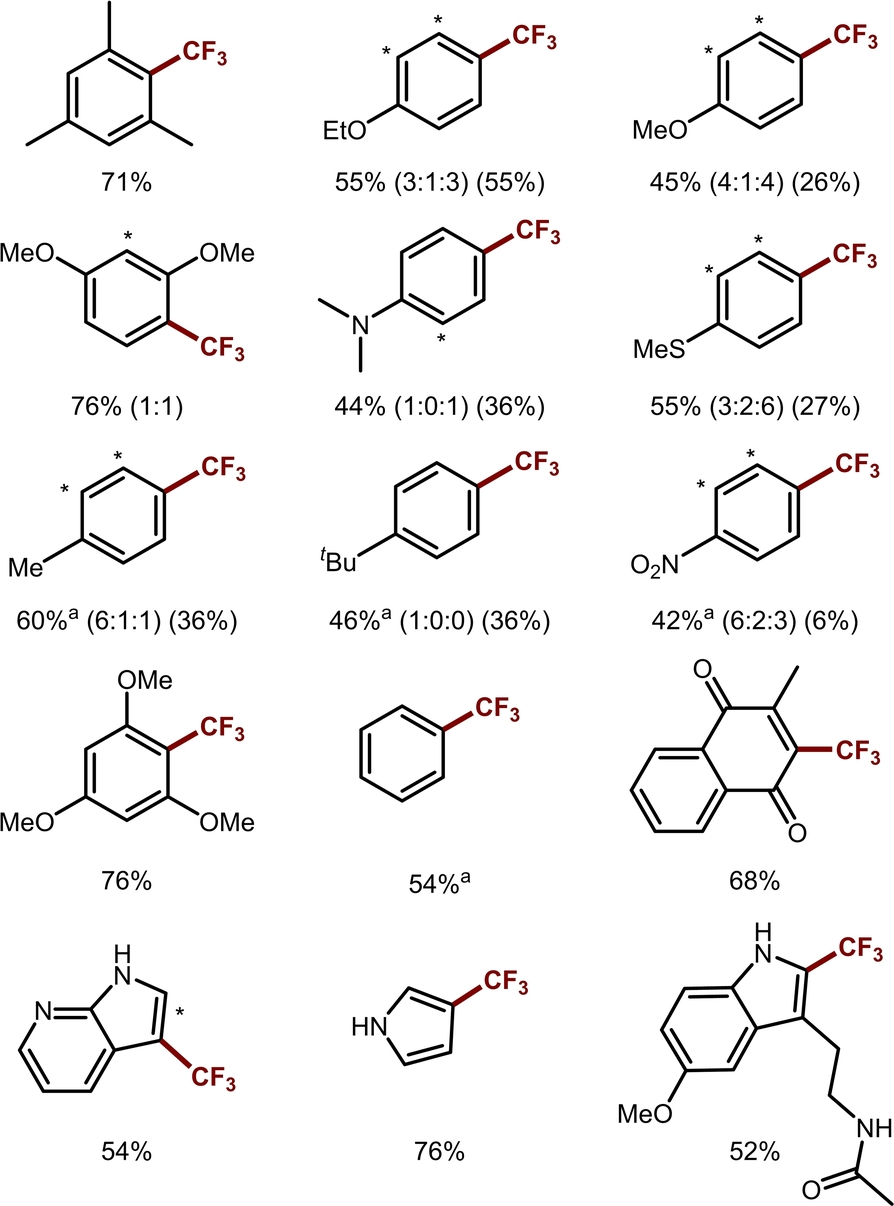
Having established the optimized conditions, the scope of C(sp2)−H trifluoromethylation was evaluated (Table 2). Overall, electron-rich (hetero)arenes show higher yields than more electron-poor substrates. The selectivity of C(sp2)−H trifluoromethylation is consistent with literature precedent.47 For tert-butylbenzene, the functionalization of ortho and meta sites is reduced due to steric hindrance. Drug-like molecules, such as melatonin, can also be successfully trifluoromethylated.
The Role of Single Electron Transfer in CF3 Transfer
The transfer of CF3 from LCuIIICF3 suggests that the rate-limiting step may involve the cleavage of the CuIII−CF3 bond. However, this proposal does not align with the notably accelerated rate upon the addition of oxidant. Additionally, under standard reaction conditions, electron-rich substrates exhibit significantly faster reactions (typically 5–10 minutes), while electron-poor substrates require up to 30 minutes of reaction time.
This correlation between the electronic character of the substrates and the reaction kinetics prompted our exploration into the possibility of a SET mechanism. We hypothesize that the reaction can be initiated by the oxidation of (hetero)arene substrate by [TBA]2CeIV(NO3)6 to generate an aryl radical cation intermediate, which can then be functionalized by LCuIIICF3 to afford Ar−CF3 (Scheme 3). If this proposed mechanism is followed, the rate-limiting step should be the electron transfer between the oxidant [TBA]2CeIV(NO3)6 and the arene substrate. Accordingly, kinetic analysis of the reaction between LCuIIICF3 and thioanisole indicates a first-order dependence on the concentration of the oxidant and substrate (Figure 3).
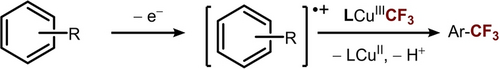
SET mechanism for C(sp2)−H trifluoromethylation.
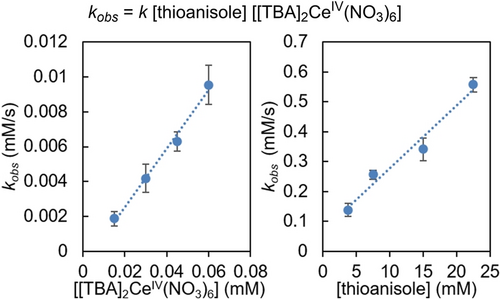
Determination of the reaction rate order dependence for [TBA]2CeIV(NO3)6 oxidant and thioanisole substrate.
To further evaluate the possibility of a SET mechanism, we conducted a Marcus study,44, 48-50 in which the driving force of electron transfer (−▵GET) and the kinetics of trifluoromethylation (k1) for a series of substituted arenes with varying electronic properties (R=H, NO2, tBu, CH3, OEt, OCH3, SCH3, N(CH3)2) are assessed. We first determined the oxidation potential (Eox) of each arene using cyclic voltammetry (CV, Figures S4–S11). Subsequently, we calculated the driving force for electron transfer with [TBA]2CeIV(NO3)6 (expressed as −▵GET) for each substrate using the equation −▵GET=−(Eox,substrate−E1/2,oxidant).51, 52
The rate of trifluoromethylation was determined for each substrate by monitoring the UV/Vis profile of LCuIIICF3 (λmax=570 nm) at 60 °C in DCE with 150 equivalents of substrate and one equivalent of [TBA]2CeIV(NO3)6. A lower temperature was chosen to ensure reliable rates were properly measured. We also determined that this set of reaction conditions is suitable for our C−H trifluoromethylation reaction (Table 1, entry 4).
The resulting first-order rate constants (k1) were used to construct a Marcus plot (lnk1 vs. −▵GET, Figure 4). If the reaction proceeds through a rate-limiting electron transfer step, we anticipate a linear correlation with slope represented by α/RT, where α is the Brønsted α value.51, 52 Interestingly, we observed two distinct regions: (1) an electron-transfer (ET) region with a linear correlation between −▵GET and lnk1 (R2=0.9985, Figure 4, red) and (2) a non-electron-transfer region (non-ET, Figure 4, blue) where the reaction rate remains similar to that of LCuIIICF3 self-decay (Figure 4, black line). The α value of ET region is modest at 0.1177 compared to the ideal value of 0.5, suggesting transition state asynchronicity caused by a delay in the product stabilization factors behind the transition state. The synchronicity of the reaction could be influenced by the aromaticity or steric effects of the arene during ET, or other molecular processes after the ET, such as the release of CF3 radical from LCuIIICF3.52, 53
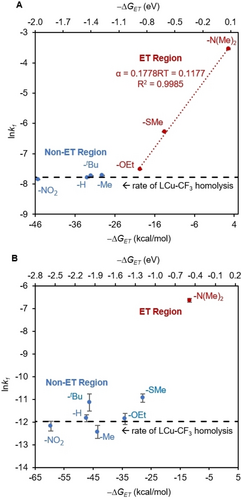
(A) Marcus plot in the presence of [TBA]2CeIV(NO3)6 oxidant. (B) Marcus plot in the absence of oxidant. The electron transfer driving force (−▵GET) is determined by cyclic voltammetry. lnk1 is determined by monitoring decay of LCuIIICF3 by UV/Vis (λmax=570 nm in DCE). All studies were performed in DCE.
The two distinct regions in the Marcus plot suggest a mechanism crossover. For electron-rich substrates, the reaction proceeds through rate-limiting electron transfer between the oxidant and arene substrate (Scheme 3). The first-order dependence on the concentration of oxidant and substrate shown in Figure 3 is also consistent with this hypothesis.
Conversely, within the non-ET region, the mechanism is likely dominated by the rate of CuIII−CF3 bond homolysis. In this case, the reaction rate is not substrate-dependent and closely resembles the self-decay rate of LCuIIICF3 (Figure 4, black line), which directly corresponds to the rate of CF3 radical release from LCuIIICF3 when the CF3 radical release/SEAr mechanism is followed (Scheme 4). Consistent with this hypothesis, EPR analysis of the products of LCuIIICF3 self-decay in MeCN at 85 °C shows the formation of LCuIIMeCN in >99 % yield (Scheme 4, Figures S58 and S59), suggesting that the decay of LCuIIICF3 quantitatively releases CF3 radical.
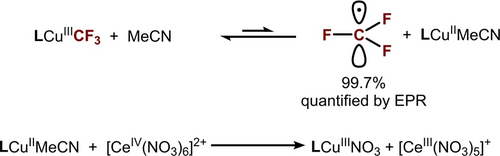
Release of CF3 radical from LCuIIICF3.
Since the redox potential of LCuIIICF3 is quite oxidizing (−0.15 V vs. Fc/Fc+), the LCuIIICF3 complex itself could potentially engage in ET with electron-rich arenes. To examine this hypothesis, we performed an analogous Marcus study in the absence of oxidant. In this case, the driving force of ET is calculated using the redox potential of LCuIIICF3 (−0.15 V vs. Fc/Fc+). Indeed, the resulting Marcus plot also suggests a mechanism crossover when the ET driving force approaches −1.0 eV (Figure 4B). While the majority of arenes exhibit a rate similar to the self-decay of LCuIIICF3, the rates of the -SMe and -NMe2 derivatives are markedly faster, suggesting electron transfer. Notably, the rate of release of CF3 radical from the LCuIIICF3 is slower in the absence of an oxidant. This is due to the further reaction of LCuII with [TBA]2CeIV(NO3)6 to generate LCuIIINO3, which drives the equilibrium of CF3 radical release to the completion (Scheme 4).
To further investigate the mechanism dichotomy, we designed a substrate, 1,2,3-trimethyl-5-(allyloxy)-benzene (Figure 5A, 1), which has a redox potential between the ET region and the non-ET region (Eox=0.927 V vs. Fc/Fc+ in DCE, −▵GET=−0.455 eV). In the case of CF3 radical release, the SEAr reaction of arene and CF3 radical is expected to produce 2, and the CF3 radical addition to the alkene is expected to produce the cyclized product 3.54, 55 However, if the SET pathway is followed, only the cyclized product 3 is expected.56 This selectivity of the SET pathway to 3 is because the oxidation of 1 takes place at the arene group rather than the allyl ether, as the oxidation potential of 3 (Eox, 0.927 V vs. Fc/Fc+) closely matches that of 4 (0.928 V vs. Fc/Fc+) and is much lower than 5 (1.210 V vs. Fc/Fc+, Figure 5B, see Supporting Information). Since the SET pathway exclusively yields compound 3, a higher selectivity for 3 is anticipated when the reaction is carried out in the presence of a strong oxidant. Indeed, when the trifluoromethylation reaction of 1 is conducted in the presence of one equivalent of [TBA]2CeIV(NO3)6 oxidant, the ratio of 2 to 3 shifts from 1.2 : 1 (without oxidant) to 1 : 1.3 (with oxidant). The flipped product distribution is consistent with the proposed mechanism dichotomy – the SET driving force is higher in the presence of [TBA]2CeIV(NO3)6, therefore increasing the contribution of SET pathway to trifluoromethylation.
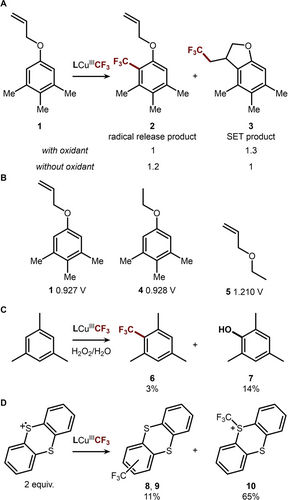
(A) Aryl radical trap experiment via intramolecular cyclization. (B) Oxidation potentials (V vs. Fc/Fc+) of aryl radical trap substrate in comparison to other model compounds. (C) The aryl radical cation intermediate can be trapped with H2O2/H2O to produce phenol. (D) Thianthrene radical cation reacts with LCuCF3 to produce trifluoromethylated products.
The SET mechanism was further tested by trapping the aryl radical cation intermediate. Trifluoromethylation of mesitylene was performed in the presence of excess amount of H2O2 (24 equiv. in 56 equiv. H2O). Under these conditions, the yield of the trifluoromethylated product 6 drops from 72 % to 3 %, while phenol product 7 is observed at a 14 % yield, suggesting the aryl radical cation intermediate is trapped with H2O2 or H2O prior to functionalization by LCuIIICF3 (Figure 5C). Finally, to evaluate whether LCuIIICF3 can capture aryl radical cations, we investigated its reaction with thioanthrene radical cation PF6 salt.57, 58 The stable radical cation serves as a model for the aryl radical cation intermediate generated via the electron transfer mechanism. Treatment of LCuIIICF3 with two equivalents of thioanthrene radical cation afforded Ar−CF3 coupled products 8 and 9 in a total 11 % yield (Figure 5D). The modest yield is attributed to competitive S−CF3 coupling, which produced 5-(trifluoromethyl)-5H-thianthren-5-ium (10) in 65 % yield. Despite the low efficiency, the successful Ar−CF3 bond formation supports the proposed coupling between radical cations and LCuIIICF3 complex.
Conclusions
In summary, we have synthesized and characterized formal CuII and CuIII monotrifluoromethyl complexes as a platform to investigate the mechanism of copper-mediated C(sp2) trifluoromethylation. The LCuIIICF3 complex can perform trifluoromethylation of a wide range of (hetero)arenes. Marcus studies suggest a mechanism crossover as a function of the electron transfer driving forces. For electron-rich substrates, a SET mechanism is followed, and the oxidation of the arenes occurs as the first step. In contrast, electron-poor substrates undergo a SEAr mechanism involving CF3 radical release. Our finding explains why excess oxidant is necessary for efficient CF3 transfer, as is the case in many previous reports of Cu-mediated trifluoromethylation. The SET mechanism might be present in other copper-catalyzed C(sp2)−H functionalization reactions. This will be the topic of our future investigations.
Supporting Information
The authors have cited additional references within the Supporting Information.[59--79]
Acknowledgments
This material is based on work supported by the U.S. National Institute of Health (NIH) under award number R01-GM145746 (SZ) and the National Science Foundation under award number CHE 1955284 (NKS). We thank Cheyanne Laux for her preliminary synthetic efforts.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.