B,N-Embedded Helical Nanographenes Showing an Ion-Triggered Chiroptical Switching Function
Abstract
Intramolecular oxidative aromatic coupling of 3,6-bis(m-terphenyl-2’-yl)carbazole provided a bis(m-terphenyl)-fused carbazole, while that of 3,6-bis(m-terphenyl-2’-yl)-1,8-diphenylcarbazole afforded a bis(quaterphenyl)-fused carbazole. Borylation of the latter furnished a B,N-embedded helical nanographene binding a fluoride anion via a structural change from the three-coordinate boron to the four-coordinate boron. The anionic charge derived from the fluoride anion is stabilized over the expanded π-framework, which leads to the high binding constant (Ka) of 1×105 M−1. The four-coordinate boron species was converted back to the parent three-coordinate boron species with Ag+, and the chiroptical switch between the three-coordinate boron and four-coordinate boron species has been achieved via the ion recognition with the change in the color and glum values.
Circularly polarized luminescence (CPL) dyes have received considerable attention because of the potential applications to 3D displays, security inks, and optical communications.1, 2, 3 In particular, recently, the switching behavior of CPL has been actively studied to control the ON/OFF, color (wavelength), sign, and dissymmetry factor (glum) by external stimuli; e.g. acid/base, redox, metal coordination or photoirradiation.1j, 3
Helicenes, ortho-fused aromatics, have been recognized as one of the most promising CPL dyes.4, 5, 6, 7, 8, 9, 10 Although typical carbohelicenes have often shown weak fluorescence in a blue region, the incorporation of heteroatoms into helicenes (i.e. heterohelicenes) allows not only the electronic tuning but also the structural changes by external stimuli such as protonation, oxidation, and coordination, which are applicable to molecular switches.6, 7 Until now, however, limited examples of helicenes showing CPL switches have been reported.7 After the pioneering works on the chiroptical swich of bis(pyridine)-containing aza[6]helicenes by Crassous,7a-7c recently, Pieters, Champagne, Audisio, and co-workers demonstrated triaza[7]helicenes showing a pH responsible CPL switch,7d and Tanaka and co-workers reported the closed-heterohelicenes interconvertible between the monomeric and dimeric species via the chemical oxidation and photoreduction.7e
π-Extended helicenes have also been investigated because they usually exhibit redshifted chiroptical properties.8, 9 In addition, helicenes with large polycyclic aromatics have been regarded as helical nanographenes (HNGs), which can stabilize ionic charge or radical(s) over the π-frameworks. In comparison to typical planar NGs, distorted NGs with nonhexagon rings or helical frameworks have shown unique properties.11 For example, boron-embedded HNGs have been actively studied as circularly polarized organic light emitting diodes (OLEDs) or thermally activated delayed fluorescence (TADF) materials (Scheme 1a).9, 12 In addition, three-coordinate boron dyes act as ion sensors,13 and dynamic chiroptical switches are expected. Although boron-embedded HNGs showing a recognition of fluoride ion were reported very recently (Scheme 1b), chiroptical switches were not reported.9d, 9h, 14
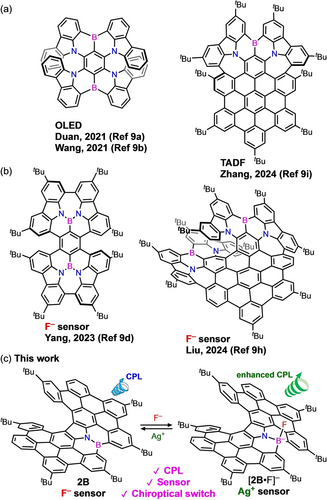
B,N-embedded HNGs showing (a) CPEL or TADF, (b) a fluoride ion recognition, and (c) CPL switches in this work.
We previously reported the synthesis of aza[7]helicenes via the intramolecular aromatic coupling of 3,6-bis(1,1’-biphenyl-2-yl)carbazoles.10 The reactions took place selectively at the 4,5-positions of the carbazoles, which were more reactive than the 2,7-positions. In the present study, 3,6-bis(m-terphenyl-2’-yl)carbazole was employed for the reaction, which afforded bis(m-terphenyl)-fused carbazole 1 (Scheme 2) via the reaction at the 2,7-positions of the carbazole as well as at the 4,5-positions. The successful synthesis of 1 encouraged us to synthesize bis(quaterphenyl)-fused carbazole 2 (Scheme 2), which was further converted into B,N-embedded HNG 2B via the borylation with BBr3. The three-coordinate boron of 2B was found to recognize F− to form four-coordinate boron species [2B ⋅ F]− (Scheme 1c). Furthermore, [2B ⋅ F]− was converted back to 2B upon the addition of Ag+, which allowed a chiroptical switch with the change in the color and glum values. Herein, we report the synthesis, structures, chiroptical properties, and switching behavior of the B,N-embedded HNG 2B. The present system enables the ion-triggered chiroptical switch in both directions, quite different from the reported systems.7
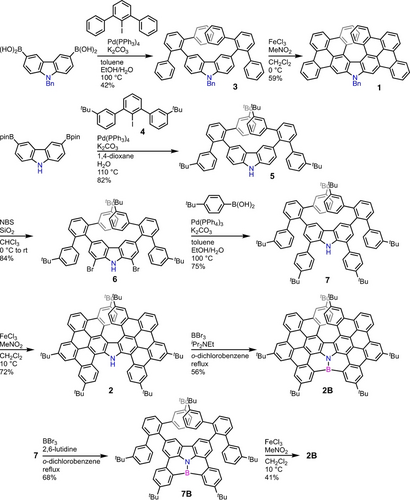
Synthesis of helical carbazoles 1, 2, and 2B.
The synthesis of 1, 2, and 2B is shown in Scheme 2. The Suzuki–Miyaura coupling of N-benzylcarbazole 3,6-diboronic acid and 2’-iodo-m-terphenyl gave 3. The intramolecular oxidative aromatic coupling of 3 with FeCl3 gave bis(m-terphenyl)-fused carbazole 1. Next, bis(quaterphenyl)-fused carbazole 2 was synthesized as follows. Six tert-butyl groups were introduced for the improvement of the solubility. The Suzuki–Miyaura coupling of carbazole 3,6-diboronic acid pinacol ester and 3,3’’-di-tert-butyl-2’-iodo-m-terphenyl (4) gave 5. Bromination at the 1,8-positions of carbazole 5 with NBS, and the subsequent Suzuki–Miyaura coupling of 6 with 4-tert-butylphenylboronic acid gave 7. The oxidative aromatic coupling of 7 with FeCl3 gave 2. Furthermore, the treatment of 2 with BBr3 in o-dichlorobenzene in the presence of iPr2NEt afforded 2B. 2B could also be synthesized via the borylation of 7 and the subsequent intramolecular oxidative aromatic coupling of 7B.
These new π-extended helical carbazoles 1, 2, and 2B were fully characterized by NMR and mass spectroscopy. Furthermore, single crystals suitable for X-ray diffraction were obtained. X-ray crystal structures clearly revealed the structures of the π-extended aza[7]helicenes (Figure 1).15 Torsion angles of the sum of the inner rim of the aza[7]helicene moieties of 1, 2, and 2B are 92.1°, 105.1°, and 85.6°, respectively. The boron atom of 2B is coplanar with the bonded three atoms, which might contribute to the somewhat smaller torsion angle. These racemic pairs of 1, 2, and 2B were successfully separated by chiral HPLC into their enantiomers showing mirror-imaged CD and CPL spectra. The absolute configuration was determined by the TD-DFT calculations of the (P)-enantiomers (Figure S1–S3 in the Supporting Information).
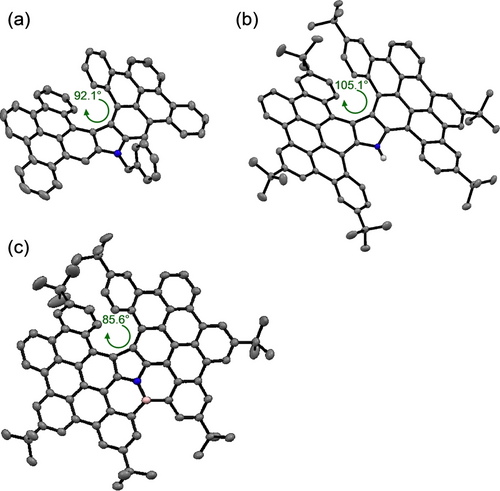
X-ray crystal structures of (a) 1, (b) 2, and (c) 2B with the thermal ellipsoids drawn at the 50 % probability level. The hydrogen atoms and solvent molecules are omitted for clarity.
Chiroptical properties of 1, 2, and 2B were investigated in CH2Cl2 (Figure 2 and Table 1). They showed relatively large molar extinction coefficients (ϵ) in the visible region, while bis(quaterphenyl)-fused carbazole 2 and 2B exhibited redshifted spectra as compared to bis(m-terphenyl)-fused carbazole 1. Upon the boron incorporation, 2B showed the somewhat larger ϵ value and Cotton effect at the longest wavelength than 2. Despite the quite similar fluorescence spectra of 2 and 2B, interestingly, the |glum| value of (P)-2 was 1.4×10−3, which was three times larger than that of (P)-2B (4.7×10−4).
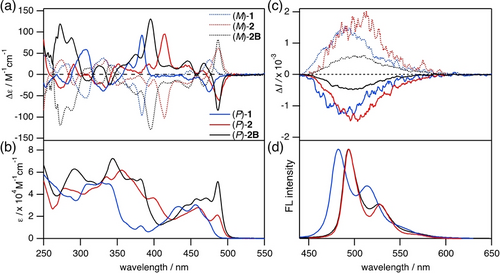
(a) CD, (b) UV/Vis, (c) CPL, and (d) FL spectra of 1, 2, and 2B in CH2Cl2.
compd |
λA [nm] |
|gabs| [×10−3][a] |
λF [nm][b] |
|glum| [×10−3][c] |
|---|---|---|---|---|
1 |
456 |
2.5 |
482, 514[d] |
1.3 |
2 |
486 |
3.0 |
494, 528[d] |
1.4 |
2B |
486 |
1.6 |
493, 526 |
0.47 |
2B ⋅ TBAF |
502 |
3.0 |
512, 545 |
1.7 |
2B ⋅ TBAOAc |
510 |
2.9 |
526 |
1.5 |
2B ⋅ TBAOH |
511 |
3.2 |
520 |
1.7 |
- [a] gabs=Δϵ/ϵ. [b] Excited at λ=360 nm. [c] glum=2(IL−IR)/(IL+IR). [d] Excited at λ=320 nm.
Cyclic voltammetry was measured, and the first oxidation potentials (Eox1) of 1, 2, and 2B were determined to be +0.61, +0.46, and +0.63 V, respectively (Table 2 and Figure S11 in the Supporting Information). On the other hand, 7B did not show oxidation waves under the measurement conditions (~1 V). DFT calculations indicated that the HOMO levels of 1, 2, 2B, and 7B were −5.03, −4.78, −5.00, and −5.41 eV, respectively, and that the LUMO levels were −1.84, −1.79, −1.98, and −1.54 eV, respectively (Figure S4 in the Supporting Information). The lower Eox1 of 2 is consistent with the destabilized HOMO level of 2. Both HOMO and LUMO levels of 2B are stabilized as compared to those of 2, while the HOMO–LUMO gaps of 2 and 2B are almost similar to each other. As the π-frameworks become larger, the HOMO–LUMO gaps decrease in the order; 7B>1>2B≈2, which is consistent with the results of the optical HOMO–LUMO gaps (ΔEopt).
compd |
Eox1 [V] |
HOMO [eV] |
LUMO [eV] |
ΔEHOMO–LUMO [eV] |
ΔEopt [eV] |
|---|---|---|---|---|---|
1 |
0.61 |
−5.03 |
−1.84 |
3.19 |
2.72 |
2 |
0.46 |
−4.78 |
−1.79 |
2.99 |
2.55 |
2B |
0.63 |
−5.00 |
−1.98 |
3.02 |
2.55 |
7B |
−[c] |
−5.41 |
−1.54 |
3.87 |
3.20 |
- [a] Determined by cyclic voltammetry; solvent: CH2Cl2, supporting electrolyte: Bu4NPF6 (0.10 M), scan rate: 0.1 Vs−1. [b] Calculated at the B3LYP/6-31G(d) level. [c] The oxidation waves were not observed at up to 1 V.
As organoboron compounds are known to adopt both three-coordinate borons and four-coordinate borons,13 2B can recognize anions to form four-coordinate boron species. When tetrabutylammonium fluoride (TBAF) was added to a solution of 2B, to our delight, clear spectral changes were observed. 1H NMR spectrum of 2B ⋅ TBAF showed 20 independent peaks for the aromatic protons because of the loss of the C2 symmetry via the axial coordination of F− (Supporting Information). In addition, 11B NMR signal for 2B was shifted from 32 ppm to 1 ppm, which is typical for four-coordinate borons by the addition of TBAF.13c In line with the structural change, the UV/Vis and FL spectra exhibited redshifted new bands (Figure 3). The binding constants (Ka) for TBAF were determined to be 1.3×105 M−1 and 9.9×104 M−1 by the UV/Vis and FL titration, respectively, with 1 : 1 curve-fitting analyses, indicating the tight binding both in the ground and excited states (Figure S7 and S8 in the Supporting Information). We also found that addition of TBAOAc and TBAOH showed the similar spectral changes with the Ka values of 1.9×103 M−1 and 5.8×103 M−1, respectively, as determined by the FL titration. The CD and CPL spectra were also redshifted, and the |gabs| and |glum| values were 3.0–3.2×10−3 and 1.5–1.7×10−3, respectively (Table 1). It should be noted that |glum| values increased more than three times upon the addition of the TBA salts.
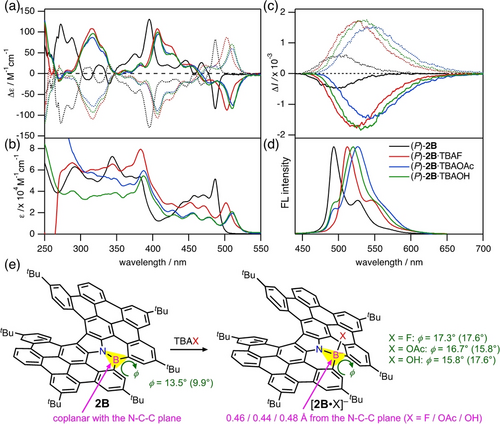
(a) CD, (b) UV/Vis, (c) CPL, and (d) FL spectra of 2B w/wo TBAX (X=F (20 eq), OAc (5000 eq), or OH (2000 eq)) in CH2Cl2. The dotted lines represent the CD and CPL spectra of the (M)-enantiomers. (e) The torsion angles of the azabora[4]helicene moieties (φ) and the distances between the boron atom and the N−C−C plane are shown based on the optimized structures. The values in parenthesis are based on the excited-state structures.
As a result of the structural changes from the three-coordinate boron to the four-coordinate borons, the optimized structures of [2B ⋅ X]− indicate that the boron atom is 0.44–0.48 Å distant from the plane of the surrounding N−C−C atoms (Figure 3e). The sum of the torsion angles of the azabora[4]helicene moieties (φ) are changed from 13.5° to 15.8–17.3° in the ground state and from 9.9° to 15.8–17.6° in the excited state. The higher planarity of 2B in the excited state (φ=9.9°) than in the ground state (φ=13.5°) likely contributed to the glum value which was much lower than the gabs value (Table 1). In fact, the glum values of (P)-2B and [(P)-2B ⋅ F]− were determined also by TD-DFT calculations to be +1.9×10−4 and −2.5×10−3, respectively, which is roughly consistent with the experimental values (Table S2 in the Supporting Information). The angle of vectors between the calculated electronic and magnetic transition moments was close to 90° for 2B, which led to the smaller |glum| value.
The high affinity of 2B to F− was considered to be due to the stabilization of the anionic charge over the π-extended carbazole moiety. We also determined the Ka value of reference compound 8B16 for TBAF to be only 4.3×103 M−1 (Scheme 3).17 The stabilization energies of 2B ⋅ TBAF and 8B ⋅ TBAF were calculated to be 38.5 and 32.9 kcal/mol, respectively, by comparing the energies before and after the binding, indicating the high stabilization for 2B ⋅ TBAF (Table S1 in the Supporting Information). The B−F bond length of 2B ⋅ TBAF is 1.50 Å, which is shorter than that of 8B ⋅ TBAF (1.52 Å). In addition, the NBO charges of the F atoms (ρF) of 2B ⋅ TBAF and 8B ⋅ TBAF were calculated to be −0.546 and −0.556, respectively. The ρF of TBAF was calculated to be −0.737, and the decrease in the negative charge as well as the above results clearly indicates that the anionic charge is effectively delocalized over the π-framework of 2B, leading to the high affinity to F−.
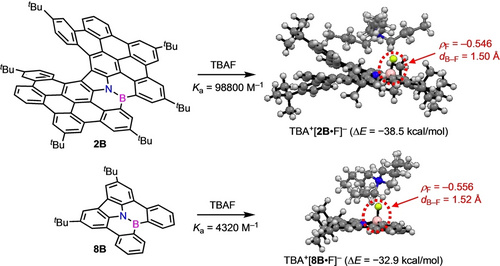
Optimized structures of 2B ⋅ TBAF and 8B ⋅ TBAF calculated at the B3LYP/6-31G(d) level. ρF and dB−F represent the NBO charges of the F atoms and B−F bond lengths, respectively.
Finally, we investigated the reversibility between the three-coordinate boron and four-coordinate boron species. Gratifyingly, the addition of AgNO3 to 2B ⋅ TBAF reproduced 2B with the color change from green to blue (Figure 4a). This finding with the change in the glum values urged us to switch the two coordinating modes. The CPL spectra confirmed that the iterative addition of 6 eq of TBAF and 6 eq of AgNO3 to a solution of (M)-2B allowed 8-time switches with the changes in the color and glum values (Figure 4b, 4c and Figure S10 in the Supporting Information).
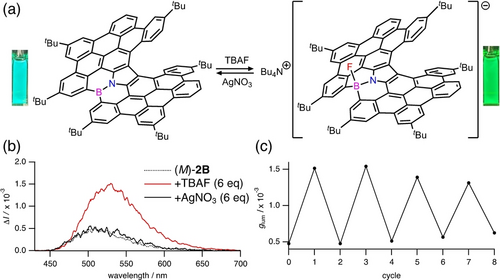
(a) Interconversion between 2B and 2B ⋅ TBAF. Photographs of the CH2Cl2 solutions under black light (λ=365 nm) are shown. (b) CPL spectral change of (M)-2B upon the addition of TBAF (6 eq) and AgNO3 (6 eq). (c) Switch of glum values for (M)-2B. Cycle 0: (M)-2B (9.2×10−6 M in CH2Cl2). Cycle 1, 3, 5, 7: after addition of TBAF (6 eq). Cycle 2, 4, 6, 8: after addition of AgNO3 (6 eq).
In summary, we have prepared bis(terphenyl)- and bis(quaterphenyl)-fused carbazoles 1 and 2 in two or four steps. Borylation of 2 produced B,N-embedded HNG 2B with a three-coordinate boron. 2B was found to recognize anions to form four-coordinate boron species such as 2B ⋅ TBAF with the Ka of 1×105 M−1. The tight binding was achieved by the stabilization of the anionic charge over the π-extended HNG framework. As the four-coordinate boron species 2B ⋅ TBAF was converted back to 2B with Ag+, 2B and 2B ⋅ TBAF are fluoride and silver ion sensors, respectively. Finally, the chiroptical switch between 2B and 2B ⋅ TBAF was demonstrated eight times via the ion recognition with the changes in the color and glum values. Boron-embedded HNGs may be applicable to chiroptical materials such as CPEL or TADF dyes,12 and further investigation on the development of novel HNGs is currently investigated in our laboratory.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 21K05039 and 24K08395. We thank Dr. S. Mori (Ehime University) for the X-ray diffraction analysis. We thank Prof. Suga and Prof. Mitsudo for CV measurements.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




