What's Next for First Row Fluorometallacycles?
Abstract
Hydrocarbon-derived metallacycles have been identified as key intermediates in a host of catalyzed transformations of unsaturated organic substrates. In contrast, our knowledge of analogous reactivity of fluorometallacycles is underdeveloped and largely confined to first row metals. Our summary of recent advances aims to inform young investigators of the exciting challenges offered by this pursuit.
1 Introduction
The synthesis and reactivity of hydrocarbon-derived metallacycles have been investigated extensively1 and they have been identified as key intermediates in a number of catalyzed transformations of unsaturated organic substrates. Well documented examples include selective ethylene oligomerization to 1-hexene,2 alkene metathesis,3 cyclooligomerization4 and ethylene dehydrodimerization to butadiene.5 Although metallacycles derived from fluorinated substrates have been known since 1961,6 significant advances in the synthesis, reactivity and catalytic applications of first row transition metal analogues have been reported over the last two decades and constitute the topic of this review. Our coverage will be limited to metallacycles with two metal-carbon bonds in which at least one of the carbons is directly bonded to one or more fluorines.
2 Three-membered Fluorometallacycles
The coordination of fluoroalkenes is most common with late metals7 and involves significant back-bonding from the metal, prompting early researchers to regard these complexes as metallacyclopropanes, involving a 2e- oxidation of the metal center.8 In the molecular structure of Ni(C2F4)(tripod), for example, the CF2 groups are bent away from the metal by 42°.9 In their discussion of the weak π-bond in C2F4 vs. C2H4, Borden et al. also pointed out the energetic preference of fluorinated carbon radicals for a pyramidal geometry.10 A more recent computational study of CuF(C2HnF4-n) quantified this favorable deformation energy and showed that differences in Cu−C bond lengths were only observed for the unsymmetrical fluoroalkenes [cf., C2H4 1.934, C2F4 1.932, C2F3H 1.939 (−CHF), 1.924 (−CF2) and two C2F2H2 isomers 1.946 (−CH2), 1.934, 1.936 (−CHF), 1.917 Å (−CF2)].11 A fragment orbital analysis by Hughes showed that this distortion in L2Ni(C2F4) lowers the alkene LUMO energy by almost 100 kJ/mol (Figure 1) and allows for an additional back-bonding interaction between filled Ni π and alkene π* perpendicular to the C−C bond, in contrast to the results for ethylene.12 A detailed metrics comparison is enabled from the molecular structure determination of CpRh(C2H4)(C2F4) (Figure 2).13
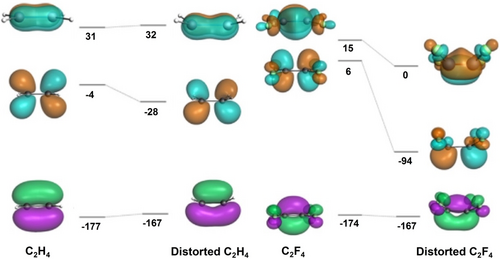
Energy shift on distortion of metal-coordinated alkenes (kJ/mol); adapted from ref. 12 with permission.
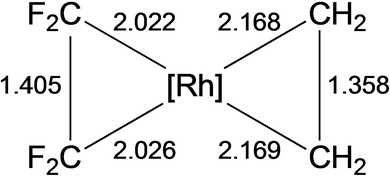
Bond distances (Å) in CpRh-bound ethene vs TFE (cf., C−C in free ethylene and TFE are 1.329 and 1.323 Å, respectively).
The synthesis of first row fluorometallacyclopropanes is typically accomplished by reaction of an electron-rich metal complex with a fluoroalkene. The extensive back-bonding ensures that coordination of a second fluoroalkene is usually more difficult, as reported for Ni atoms by Ozin and Power.14 In fact, reactions of Fe and Co carbonyl complexes with excess tetrafluoroethene (TFE) afforded instead perfluorometallacyclopentanes, as discussed in Section 3.6, 15 Besides a smattering of Co16 examples, the majority of structurally characterized first row fluorometallacyclopropanes are Ni-based and derived from a variety of fluoroalkenes and ancillary ligands (Table 1.)9, 17-24 Although these complexes are typically derived from Ni(0) precursors, Pörschke and co-workers obtained Ni(C2F4)(tmeda) (and ethane) from NiMe2(tmeda) and TFE in 65 % yield.17
Fluoroalkene |
Ancillary ligands |
Ref. |
Ni−C (Å) |
C−C (Å) |
|---|---|---|---|---|
CF2=CF2 |
tripod |
9 |
1.88(2)/1.84(2) |
1.37(3) |
CF2=CF2 |
tmeda |
17 |
1.827(3)/1.849(3) |
1.417(4) |
CF2=CF2 |
2 PCy3 |
18 |
1.9083(11)/1.9025(11) |
1.4257(15) |
CF2=CF2 |
dppf |
19 |
1.8839(14) |
1.409(3) |
CF2=CHF |
2 PPh3 |
20 |
1.888(3)/1.918(3) Disordered Ni−CF2/Ni-CHF |
1.385(4) |
CF2=CH2 |
DPEPhos |
21 |
Ni−CF2 1.824(16)/Ni−CH2 1.961(10) |
1.383(12) |
CF2=CH2 |
IAd+DMAP |
21 |
Ni−CF2 1.843(2)/Ni−CH2 1.952(3) |
1.400(4) |
CF2=CFCl |
dppe+P(OiPr)3 |
22 |
Ni−CF2 1.8970(15)/Ni−CFCl 1.9197(16) |
1.421(2) |
CF2=CF(OCF3) |
dppe+P(OiPr)3 |
22 |
Ni−CF2 1.892(2)/1.868(6)/Ni−CF(OCF3) 1.926(2)/1.948(5) |
1.400(8)/1.404(3) |
CF2=CF(CF3) |
3 PMe3 |
23 |
Ni−CF2 1.79(2)/1.91(3)/Ni−CF(CF3) 1.87(3)/1.94(3) |
1.36(3)/1.28(15) |
CF2=CH(naph) |
2 ICy |
24 |
Ni−CF2 1.864(5)/Ni−CHAr 2.007(5) |
1.435(7) |
- tripod=MeC(CH2PPh2)3; tmeda=N,N,N'N’-tetramethylethylenediamine; Cy=cyclohexyl; dppf=1,1’-bis(diphenylphosphino)ferrocene; DPEphos=bis[(2-diphenylphosphino)phenyl] ether, IAd=1,3-bis(1-adamantyl)imidazol-2-ylidene; DMAP=4-dimethylamino-pyridine; dppe=1,2-bis(diphenylphosphino)ethane; ICy=1,3-dicyclohexylimidazol-2-ylidene.
For all Ni−TFE complexes, the Ni−CF2 bonds range from 1.85–1.91 Å with P-donors, with the harder tmeda donor giving shorter Ni−C distances (1.82–1.86 Å). In all these adducts, the fluorine atoms clearly bend away from the metal, and the C−C distances in the best quality structures (1.40–1.43 Å) are significatively longer than that of free CF2=CF2 (1.323 Å).25 This indicates a strong back-donation from the metal to the alkene, as expected, consistent with Ni(II) metallacyclopropane character, although the C−C bonds are not as long as those in hexafluorocyclopropane (1.505 Å). While disorder in the structure of the Ni CF2=CHF complex does not allow us to observe the influence of F replacement by H, CF2=CH2 complexes indicate a Ni−CF2 bond significatively shorter than the Ni−CH2 bond by almost 0.1 Å, as a result of the stronger Ni−CF2 interaction. Substitution of one F of TFE by larger groups (R=Cl, OCF3, 2-naphthyl) leads to a slight lengthening of the Ni−CRF bond, but the Ni−CF2 bond remains within the range observed for unsubstituted TFE adducts. As in the above Rh example (Figure 2), comparison of the molecular structures of NiL(PCy3)2 in which L=TFE18 and ethene26 shows the former to have shorter Ni−C and longer C−C bonds (Figure S1). The same trend is observed for CpCoLL’ complexes even though the TFE example includes a PPh3 ancillary L’ ligand16 whereas that with ethylene incudes a stronger donor N-heterocyclic carbene27 (Figure S2).
2.1 Alternate Synthetic Routes
Building on the Prakash report of facile difluorocarbene generation from Me3SiCF3 and NaI,28 Baker et al. demonstrated that addition of two CF2 carbenes to a monovalent Co precursor afforded a series of fluorocobaltacyclopropanes (3 a–c, Scheme 1).16 Several Co29 and Ni30 fluorometallacyclopropanes were also derived from ring contraction of 4-membered ring analogues as detailed in Section 4.
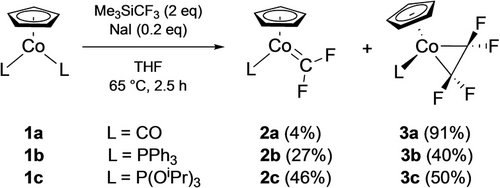
CF2 carbene route to cobaltacyclopropanes.
2.2 C−F Bond Activation
Although C−Cl oxidative addition reactions of CF2=CFCl and its metal-bound intermediates were already well known,7 the first example of fluoroalkene C−F bond activation in a first row fluorometallacyclopropane was reported by Ogoshi in 2012 (Scheme 2, right).18, 31 Conducting the thermolysis in the presence of excess TFE afforded the phosphorane, suggesting that phosphine dissociation may occur under these conditions. Indeed, no C−F bond activation was observed under the same conditions using the chelating bis(phosphine) analog, Ni(C2F4)(dcpe) [dcpe=1,2-bis(dicyclohexylphosphino)ethane]. They also showed the reaction to proceed at room temperature using stoichiometric Lewis acids such as LiI or BF3, yielding fluorovinyl products 4 b,c (Scheme 2, left). A subsequent study by Li and co-workers showed that Lewis acids effected the selective C2−F activation of Ni-bound hexafluoropropene (HFP) giving alkenyl complexes 5 a,b (Scheme 3, top).23 A similar result was obtained using a strong Brønsted acid (presumably affording HF), whereas weaker acetic acid protonated the Ni−C2 bond yielding Ni fluoroalkyl 6 (Scheme 3, bottom). In transmetallation reactions of 5 a, a double C−F bond activation was proposed to involve con-secutive substitution and elimination reactions, reflecting the enhanced fluoroalkene C−F bond reactivity upon coordination to the electron-rich Ni center (Scheme 4).
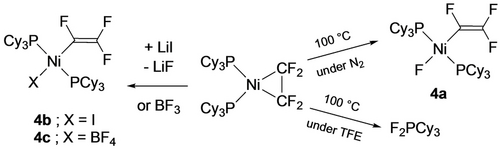
Ni-mediated C−F bond activation of TFE.
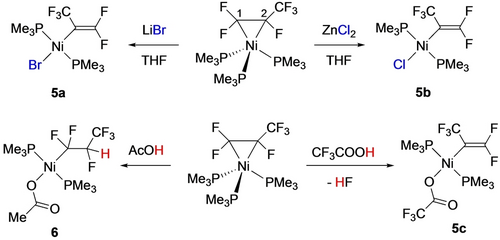
Ni-mediated C−F bond activation of HFP.
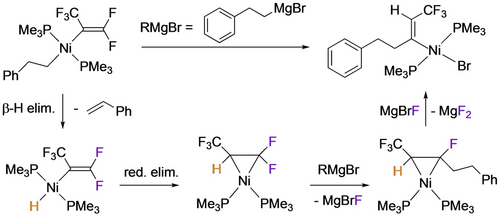
Ni-mediated double C−F bond activation.
3 Five-membered Fluorometallacycles
Although Stone and co-workers reported that low-valent metal complexes react with TFE to afford 5-membered fluorometallacycles with Fe6 and Co15 in the early 1960’s, the synthesis and chemistry of these complexes has focused largely on Ni, as shown by the structurally characterized examples (Table 2).32-49 Work by the Stone50 and Ogoshi18 groups showed that conversion of the initially formed nickelacyclopropane to its five-membered analogue was largely dependent on steric factors (Scheme 5). Most NiII[−(CF2)4−] complexes adopt a 4-coordinate square planar geometry, in which the perfluoroalkyl ring binds with nearly perfect bite angles ranging from 84 to 89°. The Ni−C bonds range from 1.87 to 1.98 Å, depending on the distortion around the metal and the nature of the trans-ligand. As a result, the T-shaped tricoordinate Ni[−(CF2)4−](SIPr) has a strong discrepancy between the bond trans to the NHC carbon [1.934(2) Å] and the one with no trans ligand [1.875(2) Å].40 A similar difference is seen with the [Ni(CF3)[−(CF2)4−](NCMe)]− anion, confirming the large trans effect of the CF3 ligand.39 The fluorinated ring also stabilizes high oxidation states with examples of Ni(III) and Ni(IV) reported by Vicic,41 Sanford,45 Ogoshi,48 and the Baker group (Figure S6).46, 47 In these octahedral complexes, however, the Ni−C bonds are slightly longer with values ranging from 1.96 to 2.01 Å. This lengthening is also observed in cationic or neutral octahedral Co(III) complexes, although the trans influence impacts inequivalent Co−CF2 bonds.34, 35 A comparison of M−C bond lengths in [M](C4F8) and [M](C4H8) complexes is shown in Figures S3–5 for M=Fe, Co and Ni.
Fluoroalkenes |
M |
Ancillary ligands |
Ref. |
M−C (Å) |
|---|---|---|---|---|
2 CF2=CF2 |
Fe |
4 CO |
32 |
2.024(3)/2.010(3) |
2 CF2=CF2 |
Fe |
3 CO+PPh3 |
32 |
1.991(9)/2.008(8) |
2 CF2=CF2 |
Fe |
CO+terpy’ |
33 |
Fe−C equatorial 1.992(3)/Fe−C axial 2.023(3) |
2 CF2=CF2 |
Coa |
4 NCMe |
34 |
1.945(3)/1.947(3) |
2 CF2=CF2 |
Coa |
terpy+NCMe |
35 |
1.978(7)/1.951(7) |
2 CF2=CF2 |
Coa |
2 bipy |
35 |
1.961(8)/1.987(8) |
2 CF2=CF2 |
Coa |
2 pym-PPh2 |
35 |
1.939(3)/1.969(3) |
2 CF2=CF2 |
Co |
terpy+Br |
35 |
1.979(2)/1.947(2) |
2 CF2=CF2 |
Ni |
2 PPh3 |
18 |
1.914(2)/1.980(2) |
2 CF2=CF2 |
Ni |
2 PEt3 |
36 |
1.948(6)/1.948(6) |
2 CF2=CF2 |
Nib |
Ph2P−Ph−S− |
37 |
1.925(6)/1.943(6) |
2 CF2=CF2 |
Ni |
2 py |
37 |
1.883(3)/1.883(3) |
2 CF2=CF2 |
Nib |
Imine,amide |
38 |
1.908(3)/1.914(3) |
2 CF2=CF2 |
Ni |
Imine,amine |
38 |
1.899(3)/1.902(3) |
2 CF2=CF2 |
Nib |
MeCN, CF3− |
39 |
1.87(2)/1.94(2) |
2 CF2=CF2 |
Nib |
MeCN, CF2CF3− |
39 |
1.897(2)/1.933(2) |
2 CF2=CF2 |
Ni |
SIPr |
40 |
1.8746(15)/1.9341(14) |
2 CF2=CF2 |
Ni |
SIPr+OH2 |
40 |
1.8868(14)/1.9257(14) |
2 CF2=CF2 |
Ni |
ItBu+P(OiPr)3 |
40 |
1.945(2)/1.956(2) |
2 CF2=CF2 |
Ni |
Κ2-terpy |
41 |
1.909(10)/1.918(10) |
2 CF2=CF2 |
Ni |
Κ1-terpy+MeCN |
41 |
1.895(11)/1.908(11) |
2 CF2=CF2 |
Ni |
Κ2-terpy |
41 |
1.901(4)/1.918(4) |
2 CF2=CF2 |
Ni |
Ph2P−PhN(H)Ph |
42 |
1.903(2)/1.921(2) |
2 CF2=CF2 |
Nib |
Ph2P−Ph−N(Ph)− |
42 |
1.905(3)/1.946(3) |
2 CF2=CF2 |
Ni |
2 MeCN |
43 |
1.887(5)/1.893(5) |
2 CF2=CF2 |
Nib |
2 MesNC |
43 |
1.935(5)/1.937(5) |
2 CF2=CF2 |
Ni |
Imine+PPh3 |
44 |
1.912(2)/1.943(2) |
2 CF2=CF2 |
Nic |
Tp− |
45 |
1.942(2)/1.974(2) |
2 CF2=CF2 |
Nic |
bipy, Cl− (dimer) |
46 |
1.972(2) trans to Cl/1.985(2) trans to N |
2 CF2=CF2 |
Nic |
SIPr, (OAc)− |
47 |
1.934(7) trans to O/1.961(8) trans to CSIPr |
2 CF2=CF2 |
Nid |
Κ3-terpy+MeCN |
41 |
1.960(4)/1.966(4) |
2 CF2=CF2 |
Nie |
2 py+2 F− |
41 |
1.972(2)/1.972(2) |
2 CF2=CF2 |
Nie |
Tp−+Aryl |
45 |
1.967(2)/1.978(2) |
2 CF2=CF2 |
Nie |
Tp−+Alkyl |
45 |
1.979(2)/1.986(2) |
2 CF2=CF2 |
Nie |
Tp−+ArCOO− |
45 |
1.999(8)/2.010(8) |
2 CF2=CHF (head-head) |
Ni |
2 PMe3 |
20 |
1.937(4)/1.1937(4) |
CF2=CF2+CH2=CH2 |
Ni |
2 PPh3 |
48 |
Ni−CF2 1.920(3)/Ni−CH2 1.942(3) |
CF2=CF2+PhCH=CH2 |
Ni |
dmeda |
49 |
Ni−CF2 1.892(2)/Ni−CHPh 1.986(2) |
CF2=CF2+PhCH=CH2 |
Ni |
bipy |
49 |
Ni−CF2 1.867(5)/Ni−CHPh 1.975(5) |
CF2=CF2+PhCH=CH2 |
Ni |
2 BnNH2 |
49 |
Ni−CF2 1.871(5)/Ni−CHPh 1.973(5) |
2 CF2=CFCl (tail-tail) |
Ni |
dppe |
22 |
Ni−CHCl 1.929(2)/1.935(2) |
2 CF2=CF(OCF3) (tail-tail) |
Ni |
2 P(OiPr)3 |
22 |
Ni−CF(OCF3) 1.942(2)/1.945(2) |
2 CF2=CF(OCF3) (tail-tail) |
Ni |
dppe |
22 |
Ni−CF(OCF3) 1.939(3)/1.942(3) |
2 CF2=CF(OCF3) (tail-tail) |
Ni |
Κ3-Xantphos |
22 |
Ni−CF(OCF3) eq. 1.964(2)/Ni−CF(OCF3) ax.1.903(2) |
- a cations as PF6− salts; b anionic as [(crown)Na]+ or Me4N+ salts; c neutral Ni(III) complex; d cationic Ni(III) complex of BF4− ; e neutral Ni(IV) complexes. dmeda=N,N-dimethylethylenediamine; terpy=2,2’:6’,2”-terpyridine; terpy’=4-p-tol-terpy; bipy=2,2’-bipyridine; pym=pyrimidine; Tp=tris(pyrazolyl)borate; SIPr=1,3-bis-(2,6-diisopropylphenyl)-2-imidazolidinylidene; Xantphos=(9,9-dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphine).
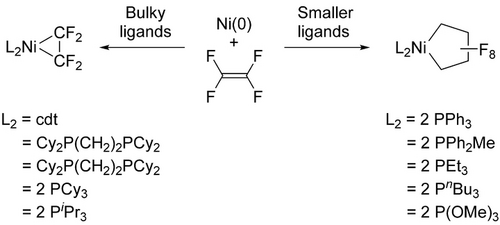
Phosphine steric effects on 5-membered ring formation (cdt=t,t,t-1,5,9-cyclododecatriene; Cy=cyclohexyl).
Several substituted fluoronickelacyclopentanes have been prepared by nucleophilic substitution at Cα (with PR3, pyridine or OAc−): in those cases (not included in Table 2), the size of the nucleophile induces a slightly longer M−CF(Nuc) bond (vs. M−CF2) by 0.04 Å on average. Other α-substituted rings have been prepared by tail-tail oxidative cyclization of functionalized fluoroalkenes CTFE and PMVE,22 where substitution only increases Ni−CFX (X=Cl, OCF3) by 0.01 Å. Mixed metallacycles from the insertion of non-fluorinated alkenes into TFE-nickelacyclopropanes have also been prepared (Section 3.2). The shorter Ni−CF2 distance (Figure 3) reflects multiple bonding interactions51 not present in Ni−CH2 and the longer Ni−P distance trans to −CF2 confirms its larger trans effect vs −CH2. With styrene, the regioselectivity of the insertion (Ph on Cα) imposes a strong elongation of the Ni−CHPh bond (vs. Ni−CF2) of more than 0.08 Å.49
Bond distances (Å) in mixed ethylene-TFE nickelacyclo-pentane.
The stability of 5-membered fluoronickelacycles is particularly notable. Although they cannot be prepared directly from ethylene, phosphine Ni(C4H8) metallacycles, generated at low temperatures, have been shown to decompose upon warming to 9 °C via β-H elimination or reductive elimination depending on the number of coordinated phosphine ligands.52 In contrast, many phosphine fluoronickelacyclopentanes can be heated at 100 °C overnight without change.
The lability of the PPh3, PnBu3 and P(OiPr)3 ligands have proven useful to access 5-membered analogues with alternate donors such as N, C and S. For example, N-heterocyclic carbene (NHC) ligands undergo reactions with fluoroalkenes to afford NHC fluoroalkenes53 and Pörschke and co-workers reported that perfluoronickelacyclopropanes derived from tmeda or diimine ligands did not react readily with a second equivalent of TFE.17, 54 The latter observation led Hughes, Baker et al. to pursue a mechanistic investigation of this 3- to 5-membered ring expansion that revealed several points of interest.19 First, kinetic barriers for adding the second TFE were lower for 16e- P2Ni(C2F4) than 14e- PNi(C2F4) (P=PMe3, PPh3). Second, the P−Ni−P angle widens to ca. 150 °C during the C−C bond-forming step in the perpendicular plane, initially affording a trigonal bipyramidal metallacycle 7 with one empty coordination site (seesaw geometry, Scheme 6).
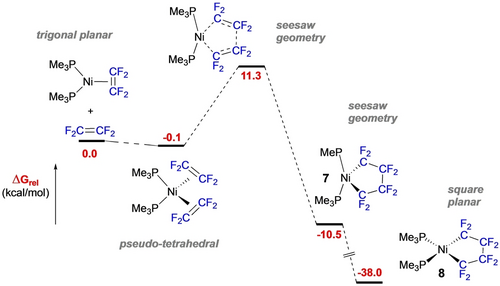
Reaction mechanism for fluoronickelacyclopentane formation.
Subsequent reaction with another ligand or donor solvent then provides a low-energy barrier to the stable square planar nickelayclopentane 8 (ca. 40 kcal/mol more stable than starting materials).
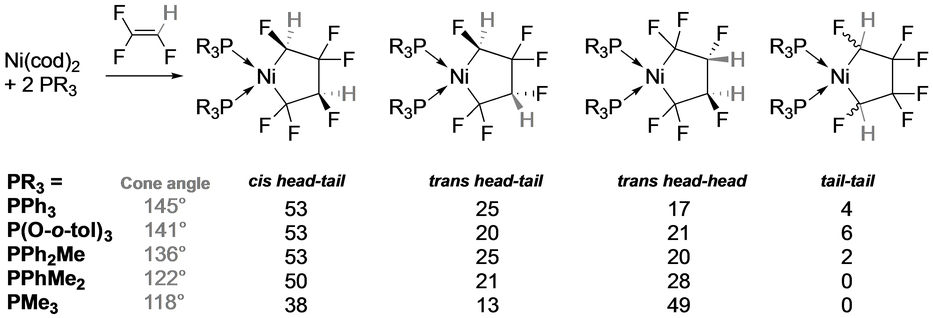
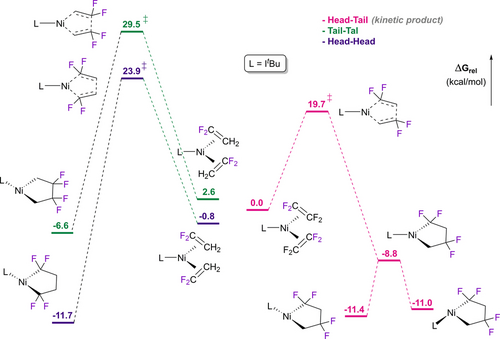
With trifluoroethene (TrFE), it was shown that the steric bulk of ancillary phosphorus ligands has an impact on the regio- and stereoselectivity of five-membered ring formation (Table 3).20 DFT studies showed that the head-head isomer is most thermodynamically stable, confirming that the regioselectivity is under kinetic control. Indeed, Stone reported that reaction of Ni(AsMe2Ph)4 with TrFE afforded the head-head isomer exclusively.55 In contrast, calculations for the reaction of Ni(ItBu) with vinylidene difluoride (VDF, CH2=CF2) shows head-head and head-tail regioisomers of similar energy whereas the cyclization transition state is much lower for the observed head-tail isomer (Scheme 7).21
Nickelacyclopentanes from VDF.
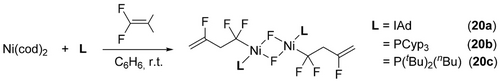
With bulkier fluoroalkene perfluoro(methyl vinyl ether) [PMVE, CF2=CF(OCF3)], reaction with Ni(cod)[P(OiPr)3]2 is regioselective for the tail-tail isomer 9 a,b with a trans:cis ratio of 3 : 1 (Scheme 8, top).22 A similar result (10) was obtained for chlorotrifluoroethene (CTFE, CF2=CFCl) although the reaction was accompanied by the C−Cl oxidative addition product 5d (Scheme 8, bottom).
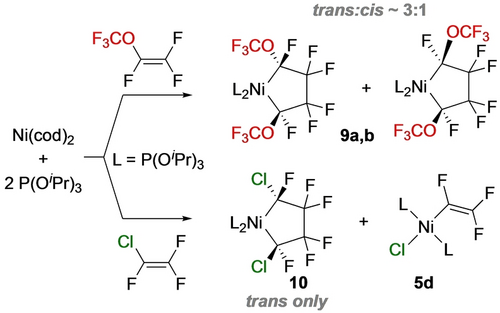
Nickelacyclopentanes from PMVE and CTFE.
3.1 Alternate Synthetic Routes
Von Werner and co-workers showed that several bis(phosphine) Ni(C4F8) complexes could be obtained from the Ni(II) dihalide precursors and NaH under a TFE atmosphere, albeit in moderate yields (20–35 %).56 Noting the hazards of working with TFE, the Vicic group developed a simple route to L2Zn[(CF2)4]2ZnL2 (11; from ZnEt2 and I(CF2)4I)57 that they used to make both d8 Ni and d6 Co perfluorometallacycles (12,13; Scheme 9).43, 34
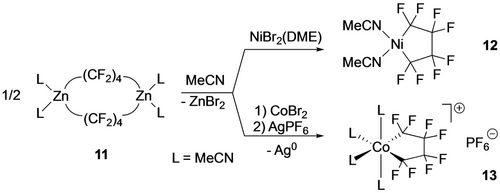
TFE-free routes to perfluorometallacyclopentanes.
3.2 Mixed Metallacycles
In their studies with N-ligated Ni precursors, Pörschke et al. showed that addition of ethene or ethyne58 to the TFE complexes afforded the mixed 5-membered metallacycles (14,15) exclusively (Scheme 10).17, 54 In contrast, Ogoshi showed that formation of the PPh3 analogue required initial addition of ethene to avoid the Ni−C4F8 metallacycle (Scheme 11).48 These authors also noted the high nucleophilicity of Cα (NBO analysis) in these mixed metallacycles which enabled their unique ring expansion chemistry and participation in Ni-catalyzed cyclizations (Section 3.4).
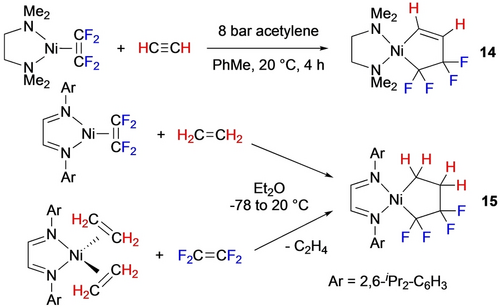
Ni-TFE mixed metallacycles with ethyne and ethene
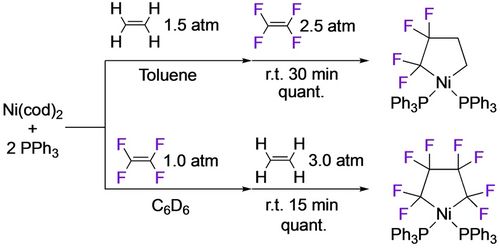
Ni-TFE-ethene mixed metallacycle.
3.3 C−F bond Activation
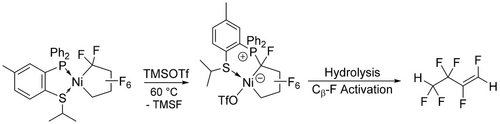
Although the formation of perfluorocyclobutene by thermolysis of Fe(C4F8)(CO)3 is presumed to involve Cβ−F bond activation, the first discrete example was due to Burch et al. (Scheme 12) using stoichiometric BF3.36 Generation of the Cα carbenium ion (or Ni fluorocarbene resonance form) is followed by phosphine migration to give the phosphonium zwitterions (16,17). Similar reactivity was reported by Baker and co-workers with a P,S ligand; hydrolysis of 18 then afforded hexafluorobutene via Cβ−F activation (Scheme 13).37 A similar reaction with the head-head isomer of TrFE-derived nickelacycle affords a bis(phosphonium) difluorobutadiene via subsequent β-F elimination (Scheme 14).20
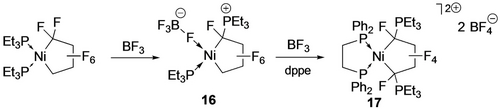
Ni-mediated Cα-F abstraction.
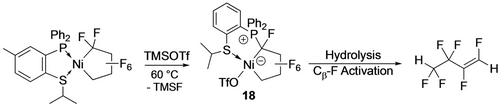
Ni-mediated hydrodefluorination.
Cα-F abstraction is followed by β-F elimination.
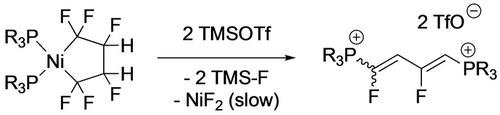
In contrast, treatment of the three-coordinate nickela-cyclopentane with TMSOTf led to ring contraction, affording the perfluorocyclobutyl complex that subsequently undergoes β-F elimination to the cyclobutene (Scheme 15).40 With the head-tail isomer of TrFE-derived nickelacycle, the observed reaction product depends on the Lewis acid.20 With BF3, ring contraction and β-F elimination yields tetrafluorocyclobutene while TMSOTf gives the aliphatic diene (Scheme 16). The latter was proposed to result from an F-shift enabled by stabilization of the Cα carbenium ion by the triflate counterion (Scheme 17).
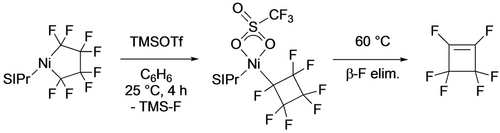
Cα-F abstraction leads to ring contraction.
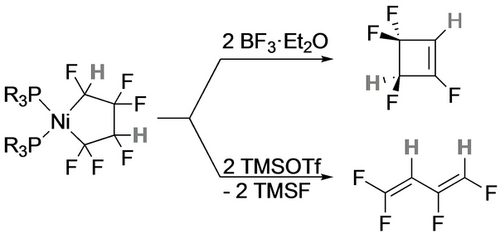
Defluorination product depends on Lewis acid.
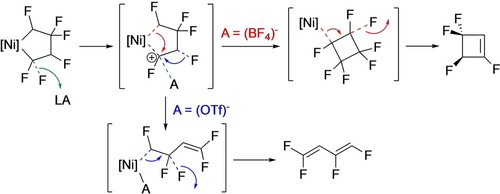
Proposed rationale for Lewis acid-derived defluorination products.
The tendency of H-containing fluoronickelacycles to undergo β-F elimination could be exploited to develop a catalytic C2→C4 fluoroalkene conversion.21 Reaction of NiL(cod) with VDF leads directly to the fluoro-bridged Ni butenyl dimer derived from the head-tail nickelacycle (Scheme 18). Transformation of Ni−F to Ni−H using a silane is followed by reductive elimination to close the hydrodefluorodimerization catalytic cycle (Scheme 19). A similar reaction employing a mixed metallacyle afforded fluorodienes from β,β-difluorostyrenes and alkynes (Scheme 20).59
Monoligated Ni precursor undergoes facile β-H elimination
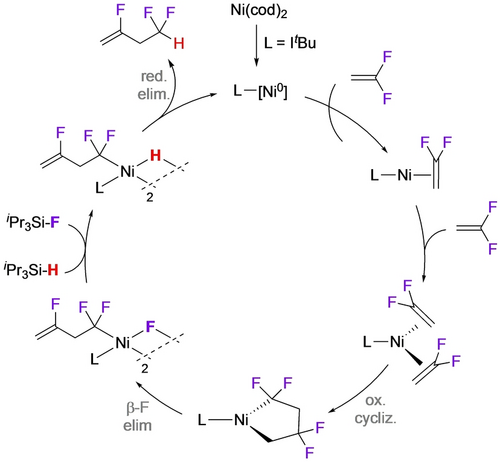
Catalytic cycle for C2→C4 fluoroalkene conversion.
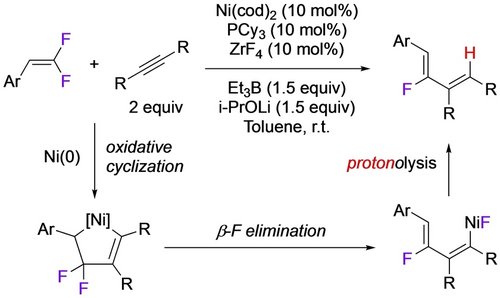
Ni-catalyzed defluorocyclization of alkynes and difluorostyrenes.
In the more Lewis acidic d6 triphos fluoroferracycle, loss of CO leads to Cα-F migration, yielding ferracyclocarbene 21, whereas the terpy’ analogue resisted CO loss, requiring a stoichiometric Lewis acid to give related product 22 (Scheme 21).33
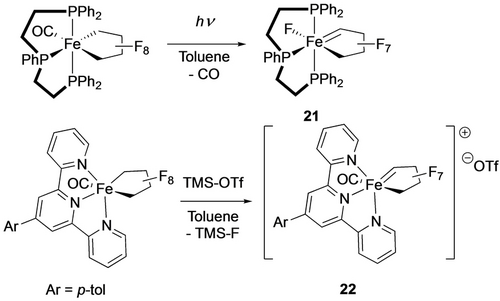
Cα-F activation in d6 fluoroferracycles.
3.4 Ring Expansion Reactions
The Ogoshi group took advantage of the high Cα nucleophilicity in tetrafluoronickelacyclopentanes, developing a number of ring expansions into catalytic cyclizations.24 Reaction of the PPh3 analogue with excess ethylene gave tetrafluorohexene, presumably via the nickelacycloheptane, followed by β-H and reductive elimination (Scheme 22).48 Utilization of the PCy3 ligand enabled a catalytic reaction with 13 turnovers. An analogous three-component coupling with TFE, ethene and benzaldehyde gave tetrafluoroketones, albeit at elevated temperature (Scheme 23).60 In a remarkable demonstration of selectivity, they extended this strategy to four-component coupling of TFE, alkyne, styrene and ethene (Scheme 24).61 Substituting the styrene for an aldehyde confirmed that initial alkyne insertion is preferred (Scheme 25).62
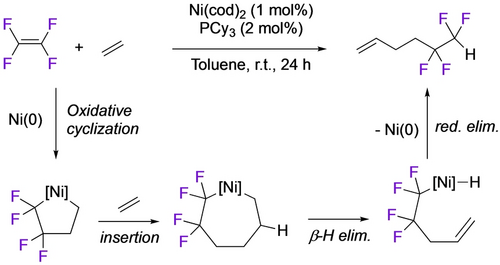
Ni-catalyzed coupling of TFE+2 ethenes.
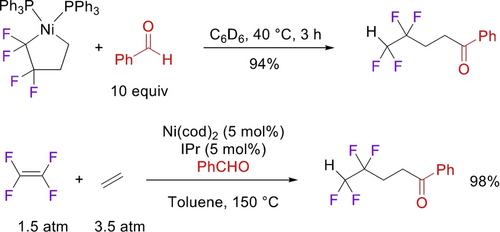
Ni-catalyzed coupling of TFE+ethene+benzaldehyde.
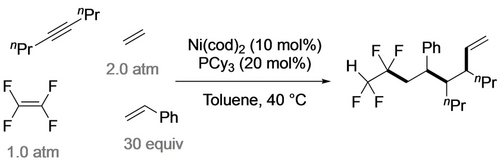
Ni-catalyzed selective coupling of TFE+alkyne+styrene+ethene.
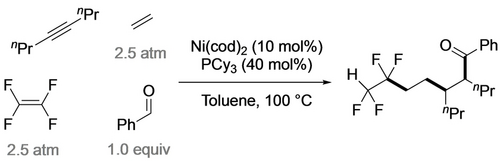
Ni-catalyzed selective coupling of TFE+alkyne+ethene+aldehyde.
4 Four-membered Fluorometallacycles
Considering the importance of 4-membered metallacycles in alkene and alkyne metathesis, the synthesis and reactivity of fluorometallacyclobutanes is particularly underdeveloped (Table 4). The first example (23) was obtained by thermal decarbonylation of an iron diacyl complex (Scheme 26, top).63
Metal |
Ligands |
Ring formula |
M−C (Å) |
Ref. |
|---|---|---|---|---|
Fe |
4 CO |
−(CF2)3− |
2.009(2)/2.009(2) |
63 |
Co |
Cp+P(OMe)3 |
−(CF2)3− |
1.938(3)–1.950(2) |
29 |
Co |
Cp+PMePh2 |
−(CF2)3− |
1.949(4)/1.952(4) |
29 |
Co |
Cp+PMePh2 |
−[CF(CF3)(CF2)2]− |
1.986(2)/1.938(2) |
29 |
Co |
Cp+PMePh2 |
−[CF(CF3)(CH=CPh)]− |
2.000(3)/1.953(3) |
67 |
Ni |
dppe |
−(CF2)3− |
1.927(2)/1.943(2) |
30a |
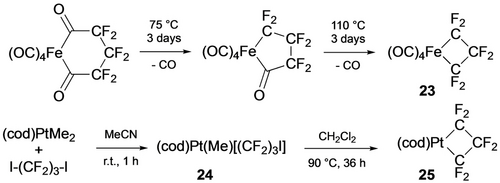
Synthesis of metallacyclobutanes.
Although the Vicic group did not report the synthesis of 4-membered metallacycles using their dizinc reagent,57 they were able to access a platinum derivative (25) from the perfluoropropyl diiodide (Scheme 26, bottom).64 More closely mimicking the metathesis pathway, Baker et al. prepared several electron-rich d8 carbene complexes, CpLCo=CFRF (RF=F, CF3), that undergo addition to tetrafluoroethene to yield cobaltacyclobutanes (27 a,b; Scheme 27).29 After noting that the slow reaction rate was not affected by excess phosphine, they teamed up with Hughes and Ess to show, using DFT methods, that the 18e- cobalt carbene undergoes electron transfer to TFE, yielding the singlet 1,4-diradical intermediate 26.65 Moreover, this reaction was especially exothermic with TFE (−25.3 kcal/mol vs −12.3 for VDF and −7.1 for ethylene). A similar pathway was reported soon after in the reaction of a divalent porphyrin-Co=CF2 complex with n-Bu-acrylate.66
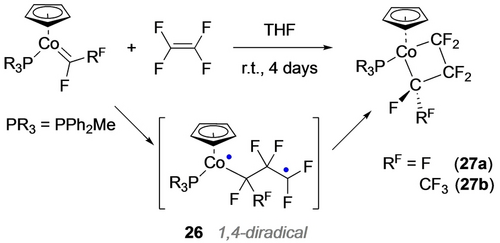
Synthesis of perfluorocobaltacyclobutanes via diradical intermediate.
Employing a more electron-rich d10 Ni perfluorocarbene, [P(OiPr)3]3Ni=CFRF, with labile phosphite ligands gave much faster reactions with TFE to form, in the case of R=CF3, both nickelacyclobutane and metathesis products (Scheme 28).30b A computational investigation by Jia and Hall showed that 16e- P2Ni=CF(CF3) is the preferred intermediate for both diradical and metathesis pathways, with the C−C bond formation in the latter occurring perpendicular to the P−Ni−P plane.
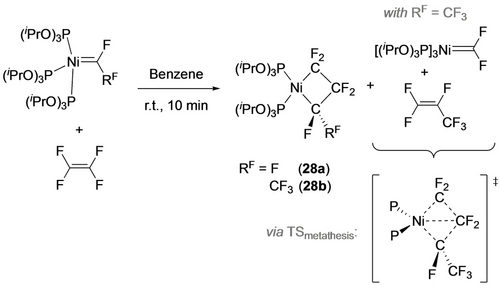
Perfluoronickelacyclobutane synthesis is accompanied by metathesis products derived from TFE coordination to Ni.
The ratio of metathesis to metallacycle products is dependent on the nature of both the fluoroalkene and the ancillary ligands. With TFE, for example, the 1 : 9 ratio with P(OiPr)3 increases to 1 : 1.4 with P(OMe)3, presumably a result of more favorable steric factors for alkene coordination with the latter. This ratio also increased with decreasing number of fluorine atoms (cf. from 1 : 9 with TFE to 1 : 5 with VDF and 1 : 3 with CHF=CF2), likely a result of increasing kinetic barriers for the diradical pathway. Surprisingly, reactions of 28 b with vinylarenes and ethylene afforded only metallacycles, in spite of the lower energy metathesis transition states.30d A comparison of 4-methoxy-styrene with the pentafluorophenyl derivative confirmed that coordination of the former to Ni is not followed by cycloreversion, allowing for an additional phosphite dissociation pathway to access the square planar metallacycle product (Scheme 29). Although reaction of 28 b with cyclopentene initially afforded the strained nickelacyclobutane (31), it underwent ring contraction via a 1,2-H shift giving 32 (Scheme 30).
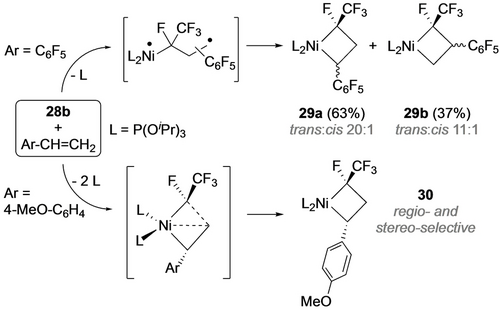
Fluoronickelacyclobutanes from styrene derivatives
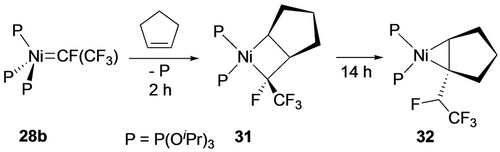
Ring contraction via H-shift.
Regioselective reaction of cobalt fluorocarbenes with terminal alkynes afforded fluorometallacyclobutenes (33 a,b) with computational studies again favouring the diradical pathway (Scheme 31, top).67 With the more electron-rich P3Ni=CF2 complex, the resulting metallacycle (34) undergoes ring contraction to nickelacyclopropene 35 via an H-shift (Scheme 31, bottom).30c
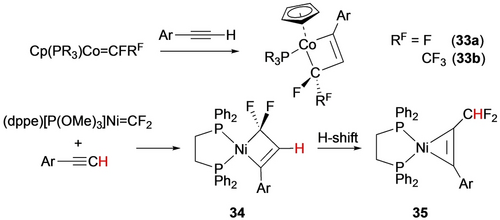
Synthesis of fluorometallacyclobutenes.
4.1 C−F Bond Activation
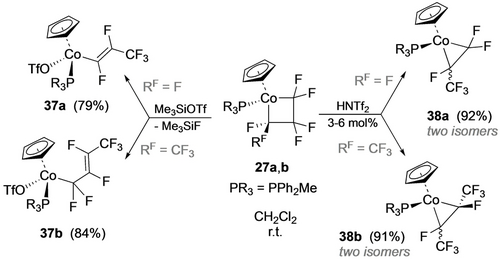
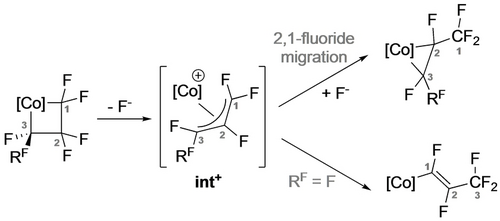
The C−F activation chemistry observed for fluorometalla-cyclobutanes differs considerably from its 5-membered analogues. Treatment of 27 a,b with stoichiometric TMSOTf afforded alkenyl complexes (37 a,b; Scheme 32).29 While the latter could arise by selective Cβ−F abstraction and selective Co−Cα bond cleavage, the former is accompanied by a formal 1,3-F shift. In contrast, reactions of 27 a,b with catalytic bistriflimidic acid gave the ring contraction products 38 a,b. The selectivity for Cβ−F abstraction in 27 a,b was attributed to formation of the Co perfluoroallyl intermediate Int+ (Scheme 33).
Fluorocobaltacyclobutane C−F bond activation reactions
Intermediacy of cobalt fluoroallyl cation.
4.2 Ring Expansion Reactions
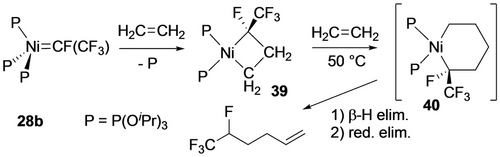
The only example of fluorometallacyclobutane ring expansion is derived from ethylene insertion into nickelacycle 39 to give nickelacyclohexane intermediate 40 (Scheme 34).30d Subsequent β-F and reductive elimination afford the tetrafluorohexene product.
Ni-mediated fluorinated alkene synthesis.
5 Other Fluorometallacycles
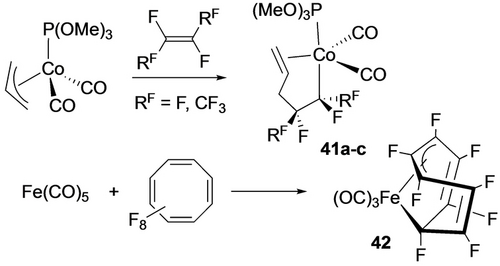
Early examples of fluorometallacycles were derived from reactions of TFE with allyl cobalt complexes (Scheme 35, top),69 a rare example of fluoroalkene insertion into an M−Csp3 bond,70 and Hughes’ work with the octafluorocyclooctatetraene ligand which affords a different isomer than the η4-diene hydrocarbon analogue (Scheme 35, bottom).71 Wilkinson's product from perfluorocycohexadiene and iron carbonyl is best described as a bent σ2-bonded η4-metallacyclopent-3-ene, halfway between a classical η4-diene complex and a metallacyclopentene (Scheme 36);72 its thermolysis to perfluorobenzene was an early example of C−F bond activation. Following up on earlier work with fluoroplatinacyclobutanes,64 Vicic prepared the first isolable example of a perfluorometallacycloheptane (43; Scheme 37).73 Employing a monoligated Ni precursor, Ogoshi et al. prepared and characterized an octafluoro analogue. (44; Scheme 38).60
Miscellaneous fluorometallacycles.
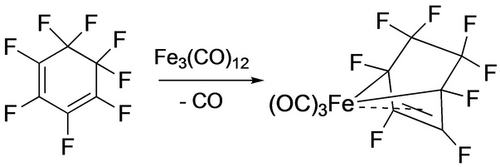
Synthesis of a perfluoroferracycle.
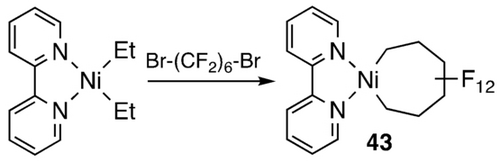
Synthesis of a perfluoronickelacycloheptane.
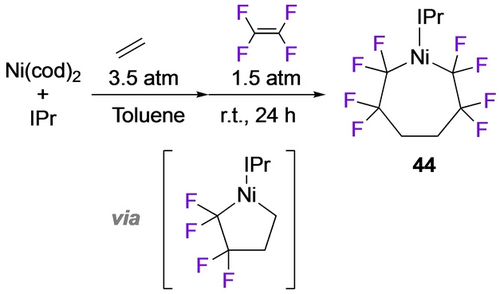
Synthesis of an octafluoronickelacycloheptane.
6 Takeaways and Future Prospects
With the discovery that hydrofluorolefins (HFOs) can serve as refrigerants, foam-blowing agents and solvents with a lower global warming potential than many previously employed fluorocarbons,74 the availability of fluoroalkenes for chemical researchers is increasing.75 Although TFE presents a deflagration hazard,76 thermolysis of fluorinated carboxylate salts in vacuo readily provides a safe 1 : 1 mixture with CO277 (Scheme 39). In another approach, Hu et al. identified conditions under which TFE could be generated in situ from Me3SiCF3.78 Moreover, convenient new protocols for selective fluoroalkene hydrodefluorination catalysis79 allow for access to a much wider variety of alkene starting materials for the synthesis of fluorometallacycles.
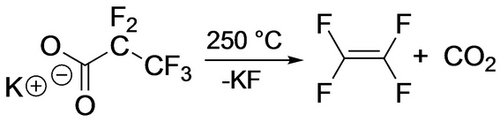
Synthesis of ‘Safe-Supply’ TFE.
Although fluorometallacycles are generally more stable than their hydrocarbon counterparts, the results summarized here show that the combination of first row metals with selected substitution of C−H for C−F bonds allows for a variety of potentially useful transformations, including C−C bond formation. Nonetheless, most reactivity studies and catalytic reactions to date have been limited to nickel. While the stability of d0 Ti−CF3 complexes80 is plagued by strong Ti−F bond formation, reactions of electron-rich d2–6 metals with fluoroalkenes may afford reactive 3- and 5-membered metallacyles81 or be fine-tuned for in situ generation of selected radicals or carbenes.
Synthesis and reactivity of mixed 5-membered fluorometallacycles benefit from their regioselective (and in some cases stereoselective) formation and proclivity to undergo β-F and β-H elimination, ring contraction and expansion, and both H- and F-shifts (a gift and a curse!). Control of these reaction pathways will depend on advancing our knowledge of metal-fluoroalkene bond energies and kinetic barriers for 3- to 5-membered ring expansion (and beyond).
Further manipulation of metal fluorocarbenes to form less stable fluorometallacyclobutanes could also open up the field of tailored fluoropolymers without reliance on electron-rich co-monomers.82
Finally, as the chemical enterprise moves toward global sustainability, will there be a continuing role for fluorochemicals? One only needs to consider their uses in modern drugs, agrochemicals and advanced materials to see their current impact. Notably, fluorochemicals are also instrumental to semiconductor manufacturing and processing; viable replacements have yet to be found. Going forward, it will be up to creative chemists and engineers to effectively minimize the environmental and ecological impact of fluorochemicals83 through the development and implementation of less persistent materials and new manufacturing processes.
Acknowledgments
RTB is indebted to his dedicated fluorine chemistry colleagues, co-workers and support staff at DuPont CR&D, Los Alamos National Laboratory and the University of Ottawa, to his generous international collaborators, and to all those who funded our investigations.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Biographical Information
R. Tom Baker obtained his BSc from UBC and PhD from UCLA with Fred Hawthorne on metallacarboranes. After 15 years at DuPont CR&D developing applications of homogeneous catalysis to fluorochemicals, TiO2 and nylon intermediates, he joined the Los Alamos National Laboratory where he led catalysis projects in multiphasic alkane functionalization and chemical hydrogen production. In 2008 Baker joined the Chemistry Department at uOttawa as Tier 1 CRC in Catalysis Science for Energy Applications and Director of the Centre for Catalysis Research and Innovation.
Biographical Information
Loïc Mangin obtained his scientific baccalaureate in Épinal, France before his BSc and PhD work at Université de Montréal with Davit Zargarian on organonickel chemistry with cyclometallated phosphinites. After a postdoctoral stint with Frank Schaper at U de M, he joined the Baker group in 2022 working on both metal SNS complexes and metal organofluorine chemistry.