Bismuth as a Z-Type Ligand: an Unsupported Pt−Bi Donor-Acceptor Interaction and its Umpolung by Reaction with H2
Abstract
Establishing unprecedented types of bonding interactions is one of the fundamental challenges in synthetic chemistry, paving the way to new (electronic) structures, physicochemical properties, and reactivity. In this context, unsupported element-element interactions are particularly noteworthy since they offer pristine scientific information about the newly identified structural motif. Here we report the synthesis, isolation, and full characterization of the heterobimetallic Bi/Pt compound [Pt(PCy3)2(BiMe2)(SbF6)] (1), bearing the first unsupported transition metal→bismuth donor/acceptor interaction as its key structural motif. 1 is surprisingly robust, its electronic spectra are interpreted in a fully relativistic approach, and it reveals an unprecedented reactivity towards H2.
Phosphanes represent one of the archetypical classes of ligands in coordination chemistry and catalysis due to their variable steric bulk and their tunable donor/acceptor properties (Scheme 1a).1 In contrast, the applicability of the heavier group 15 species as ligands towards transition metals has been facing considerable limitations.2 This is most pronounced for bismuthanes, BiR3, for which only a relatively small number of examples with R3Bi→M bonding has been reported (M=transition metal atom; R=monoanionic ligand; Scheme 1b).3 This can be tracked down to the relatively poor σ-donor properties of bismuthane ligands, because the 6s atomic orbital of the central atom is energetically low-lying due to the inert pair effect and relativistic effects.4 As an additional complication, Bi−R bonds may be readily susceptible to oxidative addition reactions with electron rich transition metal centers.5, 6 In a more recent development, bismuthanes have been designed to act as Z-type ligands, in which the bismuth center accepts electron density from the central atom, thereby adding a new dimension to the role of bismuthanes as ligands in the coordination sphere of transition metals (Scheme 1d).7-10 In this scenario, an electronegative substituent X, such as a chlorine atom, is bound to bismuth, which creates an energetically low-lying σ*(Bi−X) orbital that is readily available to be populated by electron density from an electron-rich central atom.7-9 It should be noted that the role of the bismuth species in such compounds may be interpreted to center around its Lewis acidity, a well-established field of research that has gained growing interest in recent years.11, 12-14 Current developments in bismuth-based Lewis acidity include the exploitation of geometric constraints,15 the classification of bismuth species as pronouncedly soft Lewis acids,16-18 and the plausible manifestation of cationic species as exceptionally strong Lewis acids.12-14, 16, 18, 19
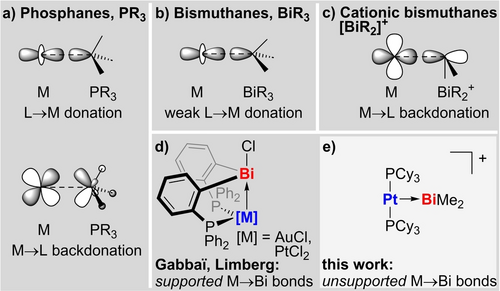
Important metal ligand interactions for phosphanes (a), bismuthanes (b), and cationic bismuthanes (c), as well as examples of bismuth compounds with supported (d) and unsupported (e) M→Bi bonds (M=transition metal).
Generally, Z-type ligands have obtained considerable interest in fields such as molecular catalysis and optoelectronics.10, 20, 21 For the very small number of literature-known bismuth-based Z-type ligands in particular, it is important to note that they are part of a more complex pincer type framework, which bears additional phosphanyl moieties.7-10 These place the transition metal in the ideal position for M→Bi bonding to be effectively realized (Scheme 1d). Unsupported M→E bonds resulting in simple dinuclear compounds, as pioneered for elements that have archetypically been exploited for Z-type ligands (e.g.: E=Al−Tl, Ag, Hg),20, 22 are unknown for bismuth. Thus, a compound with an unsupported M→Bi bonding interaction that is strong enough to persist in solution at ambient temperature would add the relevant bismuth species to the fundamentally important family of monodentate Z-type ligands (Scheme 1c, e). Notably the bismuth species would show a pronouncedly soft character, while maintaining the tunability of the steric and electronic parameters by modulation of the ligand sphere and without being connected to considerable toxicity issues - a combination that is hard to find in the classical set of monodentate Z-type ligands. Previous attempts towards compounds featuring unsupported M→Bi bonds have been hampered by excessive steric bulk around the transition metal center,23 competing ligand exchange reactions,24 and undesired oxidative addition events.5, 6
Here we report the synthesis and full characterization of [(PCy3)2Pt→BiMe2(SbF6)] and [((PCy3)2Pt)2→BiMe2(SbF6)] along with a fully relativistic interpretation of their UV/Vis spectra and reactivity studies towards H2.
In order to probe the accessibility of unsupported M→Bi dative bonding, the combination of electron-rich transition metal compounds with softly Lewis acidic bismuth cations appeared promising. Indeed, addition of one equivalent of [BiMe2(SbF6)] (I) to [Pt(PCy3)2] (II) in 1,2-difluorobenzene (DFB) instantly turns the bright yellow solution to dark blue subsequently to dark red, suggesting a sequence of reactions. The red crystalline adduct [Pt(PCy3)2(BiMe2)(SbF6)] (1) was isolated as the product of this reaction in near-quantitative yield (Scheme 2). Closer analyses, aiming to unravel the nature of the blue-colored intermediate, revealed that it only appeared when I was exposed to a superstoichiometric amount of II in weakly coordinating solvents and that its lifetime was significantly increased at low temperatures. Under optimized conditions (−30 °C, DFB), the intermediate could be isolated in 60 % yield from reactions of bismuth cation I with two equivalents of the platinum complex II and was identified as the double-dative coordination entity [(Pt(PCy3)2)2(BiMe2)(SbF6)] (2) (Scheme 2). Compound 2 is susceptible to Pt→Bi bond cleavage in solution at ambient temperature and decomposes in polar solvents such as Et2O, THF, and MeCN (to give 1 and Pt(PCy3)2 and follow-up products, depending on the conditions) and in chlorinated solvents such as DCM (through subsequent C−Cl bond activation by II). In contrast, 1 does not show any signs of decomposition when dissolved in DCM, indicating that a potential dissociation of 1 into the starting materials I and II in an equilibrium reaction does not take place to any significant extent. This is further supported by the exergonic nature of the adduct formation I+II→1 (ΔG=−33.8 kcal ⋅ mol−1), as determined by DFT calculations (Supp. Inf.). Compound 1 is also stable in THF solution, even at 60 °C, while compounds such as [Pt(PCy3)2(AlCl3)] instantly decompose in benzene solution at ambient temperature with release of II, when one equivalent of THF is added (Supp. Inf.). This behavior underlines the pronounced HSAB pair fit between softly Lewis basic Pt0 species and softly Lewis acidic bismuth monocations. Compound 1 also withstands reaction with the radical TEMPO, giving a first experimental hint at the dative character of the Pt→Bi bond (see below).
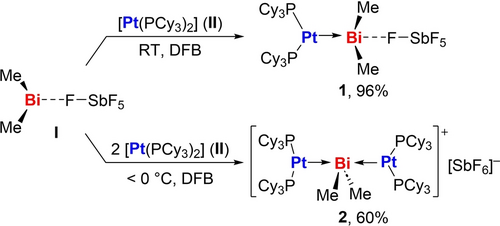
Reaction of [BiMe2(SbF6)] (I) with [Pt(PCy3)2] (II) to give the adducts 1 and 2.
NMR spectroscopic investigations reveal the expected signal patterns for bismuth-bound methyl and phosphorus-bound cyclohexyl groups. The 31P NMR spectra of 1 and 2 show resonances at 53.4 and 53.8 ppm with 1JPtP coupling constants of 2928 and 3772 Hz, which is well in line with literature-known Lewis pairs of Pt0 bases25-30, 31, 32 and rules out an oxidative addition pathway with the formation of a PtII center,5 which has previously been reported to be the dominating reaction pathway for bismuth compounds exposed to electron-rich late transition metal complexes.5, 6 The temperature-sensitivity of 2 and its limited stability in most common organic solvents required a low-temperature analysis in DFB, making a detailed comparison of 1H and 13C NMR chemical shifts with literature data less meaningful. The BiMe2 group in 1 shows chemical shifts of 1.82 ppm and 24.03 ppm in 1H and 13C spectra, respectively. This corresponds to a tremendous up-field shift compared to the free cation I (2.28/66.72 ppm), and is even similar to the parameters obtained for the bis-pyridine adduct [BiMe2(py)2(SbF6)] (1.79/33.86 ppm),17 corroborating efficient Pt→Bi bonding.
Single-crystal X-ray analyses of compounds 1 (orthorhombic space group Pca21, Z=4) and 2 (triclinic space group P , Z=2) confirmed the structural assignments based on spectroscopic data and elemental analyses (Figure 1). When excluding a small number of cluster compounds,33 for which the determination of oxidation states is less meaningful, compounds 1 and 2 are the first examples of molecular complexes with Pt0−Bi bonding interactions. In both cases the platinum atoms are coordinated by two phosphanes and the BiMe2 unit in a distorted T-shaped geometry. The phosphane ligands occupy trans-positions and the distortion is more pronounced in 2, which was ascribed to steric interactions. The bismuth atoms in both structures adopt bisphenoidal coordination geometries with a platinum atom and a weakly bound [SbF6]− anion (in compound 1) or two platinum atoms (in compound 2) in the axial positions. The Bi−Pt bond length in 1 (2.6867(2) Å) is considerably shorter than those in 2 (2.7781(6)–2.7963(6) Å), which is ascribed to the trans-influence. It is also shorter than PtII→Bi bonds that have been realized by placing the Pt center in proximity to the bismuth atom by virtue of specifically designed pincer ligands (2.7814(2)–3.0620(4) Å).8, 9, 34 The Bi−C bond lengths in 1 (2.269(5)–2.236(5) Å) and 2 (2.283(9)–2.289(9) Å) are slightly elongated compared to those in precursor I (2.215(5)–2.223(5) Å),17 and a detailed analysis of bond lengths and dihedral angles suggests weak σ(P−Pt)→σ*(Bi−C) interactions, thereby strengthening the Pt→Bi bond (Supp. Inf.).
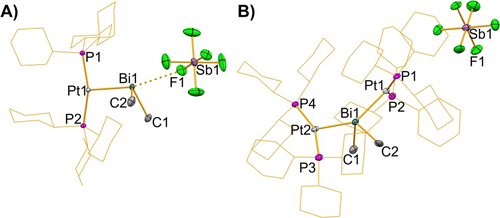
A) Crystal structure of 1, displacement ellipsoids are drawn at 50 % probability level. Hydrogen atoms are omitted and cyclohexyl-groups shown as wireframe for clarity. Selected bond lengths [Å] and angles [°]: Pt−Bi 2.6867(2), Bi−C1 2.269(5), Bi−C2 2.236(5), Bi⋅⋅⋅F 3.245(4), P1−Pt−P2 161.88(4), C1−Bi−Pt 108.04(13), C2−Bi−Pt 96.06(14), C1−Bi−C2 89.8 (2), Pt−Bi⋅⋅⋅F 165.20(7). B) Crystal structure of 2, displacement ellipsoids are drawn at 50 % probability level. Hydrogen atoms and lattice bound solvent molecules are omitted and cyclohexyl-groups shown as wireframe for clarity. Selected Bond lengths [Å] and angles [°]: Pt1−Bi 2.7781(6), Pt2−Bi 2.7963(6), Bi−C1 2.289(9), Bi−C2 2.283(9), Pt1−Bi−Pt2 145.810(16), C1−Bi−C2 88.3(3), C1−Bi−Pt1 109.9(3), C1−Bi−Pt2 99.0(3) C2−Bi−Pt1 96.8(3), C2−Bi−Pt2 102.0(3), P1−Pt1−P2 151.08(9), P3−Pt2−P4 154.81(9).35
The bonding situation in compounds 1 and 2 was further investigated by DFT calculations with a focus on the nature of the Pt−Bi interaction. Kohn–Sham molecular orbital theory together with an energy decomposition analysis (EDA) was performed using fragments from homolytic and heterolytic Pt−Bi bond splitting (Supp. Inf.). Lower absolute values of the orbital interaction energies ΔEoi were obtained for the heterolytic approach, assigning a dative Pt→Bi character to the Pt−Bi bonds in both 1 and 2. This is further supported by the analysis of both the fragments and populations of the fragments. A closer analysis of the individual attractive energy contributions (ΔVelstat and ΔEoi) also confirms the dative nature of this interaction. For 1, the electrostatic interaction ΔVelstat is similar in magnitude to ΔEoi and complemented by only relatively small dispersion contributions ΔEdisp, as might be expected in view of the small methyl substituents (ΔVelstat/ΔEoi/ΔEdisp=47 : 38 : 15). While the dative character is also apparent for the second Pt→Bi bond in 2, the relevance of electrostatic contributions and dispersion interactions is increased due to poorer overlap between fragment molecular orbitals (FMOs) and the presence of six additional cyclohexyl substituents (ΔVelstat/ΔEoi/ΔEdisp=46 : 26 : 28). For compounds 1 and 2, the FMOs responsible for the donor-acceptor Pt→Bi bonding were identified as the HOMO-2(Pt) and the LUMO(Bi), which show main contributions by the 6s(Pt) and 5d(Pt) atomic orbitals (for LUMO-2(Pt)) and 6p(Bi) atomic orbitals (for LUMO(Bi)) (Figure 2). Charge transfer from the Pt to the Bi complex fragment results in gross Mulliken populations of 1.67 and 1.83 au (HOMO-2(Pt)) and 0.51 and 0.37 au (LUMO(Bi)) for compounds 1 and 2, respectively. EDA-NOCV analysis further supports this Pt→Bi donor-acceptor interaction (Supp. Inf.).36-38 Compared to the small group of literature-known dinuclear compounds with Pt→MG bonding25-28, 39 and the even smaller group of trinuclear species with this structural motif (MG=main group element),29, 31, 32, 40 compounds 1 and 2 stick out as the only organometallic representatives, creating straightforward opportunities to expand the chemical space by tuning of steric and electronic parameters of the organic ligands. This is combined with the robust nature of the Pt→Bi bond in 1, tolerating polar solvents and elevated temperatures.
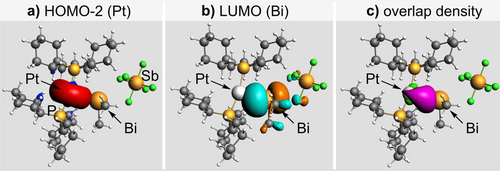
EDA of 1 (heterolytic approach). FMOs relevant for Pt→Bi bonding at an isovalue of 0.003 (a, b) and overlap density at an isovalue of 0.001 (c). Analysis of 2 gave qualitatively identical results (Supp. Inf.).
The UV/Vis spectra of intensely colored 1 (dark red) and 2 (dark blue) show bands at 437 and 527 nm (for 1) and 394, 438, 509, and 604 nm (for 2), respectively (see Supp. Inf. for details). TD-DFT calculations with different treatments of relativistic effects (scalar relativistic, perturbation approach, two-component relativistic approach) reveal that upon excitation, electron density is mainly shifted from d(Pt) non-bonding or σ(Pt−P) bonding orbitals to σ*(Pt−Bi) anti-bonding orbitals. Approaches including spin-orbit coupling allow singlet-triplet transitions to gain significant intensity. For compound 2, containing three heavy elements that are involved in the optical transitions, the two-component approach clearly delivers the most reliable results (Supp. Inf).
Aiming to explore the reactivity of 1, we turned towards dihydrogen as a substrate. The facile liberation of H2 from bismuth compounds has been reported in stoichiometric and catalytic studies.41, 42, 43 In contrast, the activation of H2 by well-defined molecular bismuth compounds has not been achieved to date.44 The incorporation of platinum—an element well-known for its ability to activate H2—into bismuth compounds thus seemed especially promising. The exposure of a solution of 1 in DFB to H2 (1 bar) led to a slow color change of the solution from dark red to yellow. Reaction monitoring by 31P NMR spectroscopy confirmed the full conversion of 1 after 7 h and the formation of two new species resonating at δ=46.4 ppm (1JPtP=2587 Hz, 3) and δ=46.0 ppm (1JPtP=2555 Hz, 4) (Scheme 3). The two species co-exist in solution at early stages of the reaction and a full transformation of 3 to 4 takes place within 6 d, along with the precipitation of a black solid. 1H NMR spectra of the reaction mixture not only show resonances due to Me and Cy groups, but also two singlets in the hydridic region at δ=−15.18 ppm (1JPtH=1445 Hz) for 3 and δ=−14.49 ppm (1JPtH=1486 Hz) for 4, which is well in line with the formation of trans-configured PtII hydride complexes in a square planar coordination geometry.45-49 The thermodynamic product of the reaction, 4, could be isolated and fully characterized (Supp. Inf. and Figure 3B), the thermally labile nature of 3 and its conversion to 4 hampered the isolation of pure samples in sufficient amounts for a full characterization. However, crystallization attempts from solutions that contain 3 as the main species gave minor amounts of single-crystalline material that was analyzed by X-ray diffraction, establishing its molecular structure (Figure 3A). The formation of compounds 3 and 4 is remarkable in that i) they originate from the first reaction of a bismuth complex with H2, ii) they represent the first examples of complexes with Bi→Pt dative bonding,50 iii) 3 is the first example of a dibismuthane acting as a σ-donor towards a transition metal Lewis acid, iv) in the reaction of 1 with H2 to give 3 and 4, the polarity of the metal-to-metal donor/acceptor interaction is inverted from Pt0→Bi with a cationic bismuth ligand in 1 to Bi→Pt2+ with neutral bismuth ligands in 3 and 4. Compounds 3 and 4 crystallized in the triclinic space group P (Z=2) and the monoclinic space group P21/n (Z=4), respectively. Expectedly, the platinum-bound hydride ligands could not unambiguously be located in the Fourier electron density map due to truncation effects and residual electron density in close proximity to the heavy atom, but their presence was proven by spectroscopic methods (see above). Taking into account the presence of one hydride ligand at each platinum center, the transition metal atoms are found in square planar coordination geometries with a trans-configuration. The platinum-bound bismuth atoms show distorted tetrahedral coordination geometries.51 The angle sums C−Bi−C/Bi in the bismuth-based ligands amount to 291° (3) and 290° (4), which indicates a considerable pyramidalization compared to the non-coordinate Bi2Et4 (282°) and BiMe3 (277°).52, 53 In the case of 3, this value even surpasses the corresponding angle sums in the only other compounds exhibiting a dibismuthane as a σ-donor ligand,54 [Bi2Et4(EtBu3)2] (E=Al 288°, Ga 286°),55 suggesting a significant hybridization of 6s and 6p bismuth atomic orbitals. The Pt−Bi bond lengths in 3 (2.7319(9) Å) and 4 (2.7030(5) Å) are slightly longer than that in the starting material 1 (2.6867(2) Å), suggesting that Pt0→BiIII dative bonding may be more efficient than BiIII→PtII bonding. Surprisingly, the Bi−Bi bond in 3 is slightly shorter than that in the free tetraalkyl dibismuthane Bi2Et4 (2.9672(6) vs 2.9827(7) Å).52 An EDA and Kohn–Sham MO analysis confirms that the Bi−Pt bonds in compounds 3 and 4 are best described as Bi→Pt donor-acceptor interactions (Supp. Inf.).
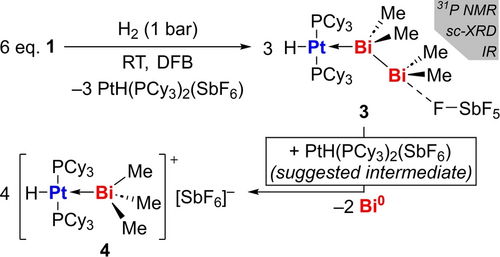
Reaction of 1 with H2 to give 3 and 4.
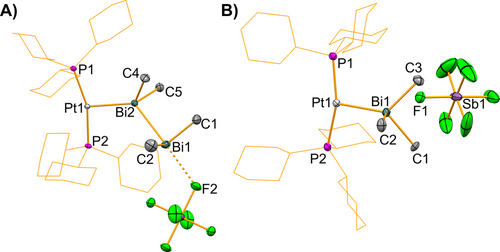
Molecular structure of 3 (A) and 4 (B). Displacement ellipsoids are drawn at 50 % probability level. Hydrogen atoms and lattice bound solvent molecules are omitted and cyclohexyl-groups shown as wireframe for clarity. The presence of one H atom in the coordination sphere of each Pt atom could not be confirmed by XRD data, but is unambiguously indicated by NMR and IR spectroscopic data. Selected bond lengths [Å] and angles [°]: 3: Pt−Bi2 2.7319(9), Bi1−Bi2 2.9672(6), Bi1−C1 2.248(7), Bi1−C2 2.259(9), Bi2−C3 2.256(7), Bi2−C4 2.256(6), Bi2⋅⋅⋅F2 2.967(5), Bi2−Bi1⋅⋅⋅F2 178.54 (10), C1−Bi1⋅⋅⋅F2 88.9(2), C1−Bi1−C2 93.2(3), C1−Bi1−Bi2 92.1(2), C3−Bi2−C4 96.8(3), C3−Bi2−Bi1 97.97(18), C3−Bi2−Pt 112.90(18), Pt−Bi2−Bi1 125.953(17), P1−Pt−P2 162.07(5). 4: Pt−Bi 2.7030(5), Bi−C1 2.231(7), Bi−C2 2.231(7), Bi−C3 2.216(7), Bi⋅⋅⋅F1 3.215(5), P1−Pt−P2 160.58(6), C1−Bi−C2 96.3(3), C1−Bi−C3 97.2(3), C1−Bi−Pt 123.8(2), C2−Bi−Pt 111.7(2), C3−Bi−Pt 125.0(2).
In order to gain deeper insights into the ligand properties of Bi2Me4 and BiMe3, IR spectra of 3 and 4 were analyzed, revealing Pt−H stretching bands at 2166 cm−1 (3) and 2184 cm−1 (4). These values are in the range of previously reported PtII hydride species and assign a trans-influence to the bismuth-based ligands that is stronger than that of MeOH or γ-picoline, and close to that of CO ( Pt−H=2308, 2237, 2170 cm−1, as determined for trans-[PtH(PCy3)2L][PF6]).45-48 In agreement with the presence of a hydride ligand in 4, this compound is EPR-silent.
The mechanism leading to the formation of 3 and 4 contains multiple elementary reactions and is certainly complex. Since 1 did not show any signs of dissociation into starting materials I and II, we tentatively suggest i) a bimetallic activation of H2,56 ii) the dehydrogenative coupling of in situ-formed HBiMe2 in the coordination sphere of Pt to form the Bi2Me4 motif,41, 43 and iii) redox disproportionation of 3 Bi2Me4 to give 4 BiMe3 and 2 Bi0,57 which appear as a ligand in 4 and as a black precipitate, respectively.
In conclusion, we have synthesized and fully characterized the first compounds with unsupported Pt→Bi bonding, [Pt(PCy3)2(BiMe2)(SbF6)] (1) and [(Pt(PCy3)2)2(BiMe2)(SbF6)] (2). This establishes the simple building block [BiMe2]+ as an effective and remarkably robust Z-type ligand in transition metal chemistry. The electronic transitions of 1 and 2 can only sufficiently be understood in a fully relativistic approach, underlining the relevance of spin-orbit-coupling in these multinuclear heavy-metal-compounds. Initial reactivity studies towards H2 yield the first H2-activation by a well-defined bismuth compound, reversible Bi−Bi coupling in the coordination sphere of Pt, and the unprecedented umpolung of Pt→Bi to Bi→Pt dative bonding. We aim to further explore these findings towards the design and application of bismuth-based Z-type ligands, bismuth catenation, and catalytic transformations involving H2.
Supporting Information
The authors have cited additional references within the Supporting Information.18, 25, 36-38, 58-61, 62-64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75-79
Acknowledgments
Financial support by the DFG (LI2860/5-1) and the LOEWE program (LOEWE/4b//519/05/01.002(0002)/85) is gratefully acknowledged. This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreements No 946184). T. E. and A. J. K. acknowledge funding from the Research Council of Finland (grant 340584) and computational resources from Finnish IT Center for Science (CSC). J. P. thanks the Spanish Ministerio de Ciencia, Innovación y Universidades (PID2022-138861NB-I00, CEX2021-001202-M), and the Generalitat de Catalunya (2021SGR442). Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




